Preclinical mouse models of immune checkpoint inhibitor-associated myocarditis
IF 10.8
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
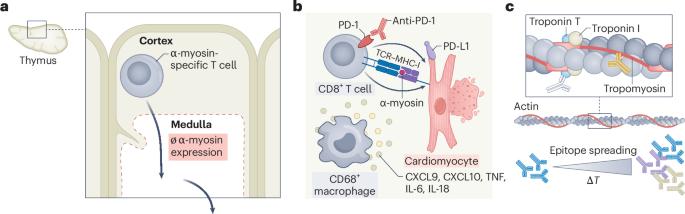
In this Review, we present a comprehensive analysis of preclinical models used to study immune checkpoint inhibitor-associated myocarditis (hereafter ICI-myocarditis), a potentially lethal immune-related adverse event. We begin by providing an overview of immune checkpoint inhibitors, highlighting how their efficacy in cancer treatment is counterbalanced by their predisposition to cause immune-related adverse events. Next, we draw from human data to identify disease features that an effective mouse model should ideally mimic. After that, we present a critical evaluation of a wide variety of existing mouse models including genetic, pharmacological and humanized models. We summarize insights gathered about the underlying mechanisms of ICI-myocarditis and the role of mouse models in these discoveries. We conclude with a perspective on the future of preclinical models, highlighting potential model improvements and research directions that could strengthen our understanding of ICI-myocarditis, ultimately improving patient outcomes. Fankhauser et al. provide an in-depth review of preclinical mouse models used to study immune checkpoint inhibitor-associated myocarditis (ICI-myocarditis). They discuss potential improvements to the field that could, in the future, strengthen our understanding of ICI-myocarditis.
免疫检查点抑制剂相关性心肌炎的临床前小鼠模型。
在这篇综述中,我们全面分析了用于研究免疫检查点抑制剂相关心肌炎(以下简称ici -心肌炎)的临床前模型,这是一种潜在的致命免疫相关不良事件。我们首先概述了免疫检查点抑制剂,强调了它们在癌症治疗中的功效是如何被它们引起免疫相关不良事件的倾向所抵消的。接下来,我们从人类数据中找出一个有效的小鼠模型应该理想地模仿的疾病特征。之后,我们提出了各种现有的小鼠模型,包括遗传,药理学和人源化模型的关键评估。我们总结了ici -心肌炎的潜在机制以及小鼠模型在这些发现中的作用。最后,我们对临床前模型的未来进行了展望,强调了潜在的模型改进和研究方向,可以加强我们对ici -心肌炎的理解,最终改善患者的预后。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


