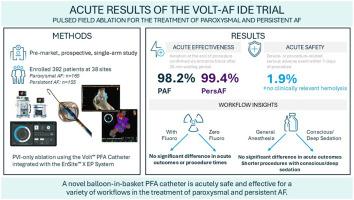
Safety and effectiveness of a novel balloon-in-basket pulsed-field ablation catheter for the treatment of paroxysmal and persistent AF: Volt-AF IDE trial acute results
IF 5.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background
Increasing use of Pulsed Field Ablation (PFA) to treat atrial fibrillation (AF) has led to concerns related to tissue contact, hemolysis, and electroanatomic mapping integration. A novel balloon-in-basket PFA catheter offers form and function to address these concerns.
Objective
The VOLT-AF Investigational Device Exemption (IDE) study is a prospective, single-arm global IDE study designed to demonstrate the Volt PFA system (Abbott Laboratories, Chicago, Illinois) is safe and effective for the treatment of paroxysmal AF (PAF) and persistent AF (PersAF).
Methods
Symptomatic, drug-refractory PAF and PersAF subjects were enrolled for de novo ablation. Ablation strategy was pulmonary vein isolation-only using the Volt PFA catheter with EnSite X EP System integration for visualization and dynamic contact display. End points were the rate of the device- or procedure-related SAE within 7 days and acute procedural success.
Results
A total of 392 subjects (57 roll-in, 335 primary analysis, 51.8% PAF, 64.7% men, age 65.0 ± 11.0 years) were enrolled at 38 sites from April to September 2024. Acute isolation was observed in 99.4% of veins (666/670) in 98.2% of patients with PAF (162/165), and in 99.8% of veins (633/634) in 99.4% of patients with PersAF (154/155), with 18.5 ± 3.6 applications/patient. Primary safety endpoint events occurred in 1.9% of subjects. Procedural efficiency and acute outcomes did not differ with fluoroscopy use. Conscious or deep sedation was associated with increased procedural efficiency, with no difference in acute success compared with general anesthesia. No clinically relevant hemolysis or kidney injury was reported.
Conclusion
These results demonstrate the acute safety and effectiveness of a novel balloon-in-basket PFA catheter to treat paroxysmal and persistent AF. Long-term outcome follow-up is ongoing.
一种新型球囊内脉冲场消融导管治疗阵发性和持续性房颤的安全性和有效性:VOLT-AF IDE试验急性结果。
背景:脉冲场消融(PFA)治疗心房颤动(AF)的应用越来越多,这引起了与组织接触、溶血和电解剖定位整合相关的关注。一种新型球囊式PFA导管提供了解决这些问题的形式和功能。目的:Volt -AF IDE研究是一项前瞻性单组全球IDE研究,旨在证明Volt PFA系统对于治疗阵发性(PAF)和持续性房颤(PersAF)是安全有效的。方法:选取有症状、药物难治性PAF和PersAF患者进行从头消融。消融策略是仅使用Volt PFA导管和EnSite X EP系统集成进行可视化和动态接触显示的肺静脉隔离。终点是7天内与设备或手术相关的SAE发生率和急性手术成功率。结果:2024年4 - 9月在38个试验点共入组392例受试者,其中入组57例,初步分析335例,PAF 51.8%,男性64.7%,年龄65.0±11.0岁。98.2% PAF患者(162/165)中99.4%的静脉(666/670)出现急性分离,99.4% PersAF患者(154/155)中99.8%的静脉(633/634)出现急性分离,每例患者18.5±3.6次。主要安全终点事件发生在1.9%的受试者中。手术效率和急性结果与透视检查的使用没有差异。清醒或深度镇静与手术效率的提高有关,与全身麻醉相比,急性成功率没有差异。无临床相关的溶血或肾损伤报告。结论:这些结果证明了新型球囊内PFA导管治疗阵发性和持续性房颤的急性安全性和有效性。长期结局随访正在进行中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Heart rhythm
医学-心血管系统
CiteScore
10.50
自引率
5.50%
发文量
1465
审稿时长
24 days
期刊介绍:
HeartRhythm, the official Journal of the Heart Rhythm Society and the Cardiac Electrophysiology Society, is a unique journal for fundamental discovery and clinical applicability.
HeartRhythm integrates the entire cardiac electrophysiology (EP) community from basic and clinical academic researchers, private practitioners, engineers, allied professionals, industry, and trainees, all of whom are vital and interdependent members of our EP community.
The Heart Rhythm Society is the international leader in science, education, and advocacy for cardiac arrhythmia professionals and patients, and the primary information resource on heart rhythm disorders. Its mission is to improve the care of patients by promoting research, education, and optimal health care policies and standards.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


