Enhancing azo dye degradation using dual enzyme systems immobilized on a mesoporous silica scaffold
IF 2.9
4区 生物学
Q3 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Highly efficient degradation of the azo dye methyl red has been achieved through a coupled enzymatic reaction using mesoporous silica as an immobilization scaffold for two different enzymes. The dispersion of the non-immobilized free azoreductase (AzoR) and glucose dehydrogenase (GDH) in the reaction solution caused vigorous aggregation of these heterologous enzymes, resulting in their deactivation. In the present study, we aimed to overcome this limitation by fusing Si-tag, a silica-binding protein, to both enzymes and simultaneously immobilizing them on the surface of highly ordered pores of mesoporous silica. Consequently, the degradation ratio of methyl red significantly increased with immobilization on the mesoporous silica compared to that from immobilization on a non-porous silica support. However, the immobilization of the AzoR and GDH with and without the Si-tag, respectively, on silica markedly decreased the enzyme activity during the reusability test owing to the desorption of GDH involved in coenzyme regeneration. By contrast, the activities of the two enzymes immobilized on the mesoporous silica surface markedly increased upon fusion of both with Si-tag. Furthermore, these dual enzyme–mesoporous silica composites prepared at high salt and surfactant concentrations exhibited higher durability and repeatability during methyl red degradation than when using non-porous silica.
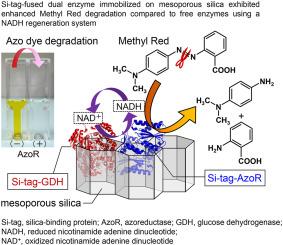
介孔二氧化硅支架固定化双酶系统增强偶氮染料降解。
采用介孔二氧化硅作为两种不同酶的固定支架,通过耦合酶促反应实现了偶氮染料甲基红的高效降解。非固定化的游离偶氮还原酶(AzoR)和葡萄糖脱氢酶(GDH)在反应溶液中的分散引起这些异源酶的剧烈聚集,导致它们失活。在本研究中,我们的目标是克服这一限制,通过融合Si-tag(一种硅结合蛋白)到这两种酶上,并同时将它们固定在介孔二氧化硅的高度有序孔表面。因此,与固定在无孔二氧化硅载体上相比,固定在介孔二氧化硅载体上的甲基红降解率显著提高。然而,在重复使用试验中,分别将AzoR和GDH固定在二氧化硅上,由于GDH的解吸参与了辅酶的再生,因此在无si标签的情况下,酶的活性明显降低。而固定在介孔二氧化硅表面的两种酶在与Si-tag融合后活性显著提高。此外,在高盐和表面活性剂浓度下制备的双酶-介孔二氧化硅复合材料在甲基红降解过程中表现出比使用无孔二氧化硅时更高的耐久性和可重复性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of bioscience and bioengineering
生物-生物工程与应用微生物
CiteScore
5.90
自引率
3.60%
发文量
144
审稿时长
51 days
期刊介绍:
The Journal of Bioscience and Bioengineering is a research journal publishing original full-length research papers, reviews, and Letters to the Editor. The Journal is devoted to the advancement and dissemination of knowledge concerning fermentation technology, biochemical engineering, food technology and microbiology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


