Lysine source for ε-poly-l-lysine biosynthesis depends on diaminopimelate pathway during its production in Streptomyces albulus
IF 2.9
4区 生物学
Q3 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Streptomyces albulus NBRC14147 produces the polycationic homopoly(amino acid) ε-poly-l-lysine (ε-PL). Due to its antimicrobial properties, nontoxicity to humans, biodegradability, and permeability, there is a high demand for ε-PL. As ε-PL is produced by l-lysine polymerization, elucidating the source of l-lysine for ε-PL production is crucial for enhancing its yield. In actinobacteria, l-lysine is produced by diaminopimelate (DAP) pathway. In this study, 2,6-pyridine-dicarboxylate (PDC) was identified as the inhibitor of DapB, a DAP pathway enzyme, by comparing the structure of DapB from Mycobacterium tuberculosis with the model structure of DapB from S. albulus. We also found that adding PDC inhibited the growth of S. albulus. More importantly, PDC additions during the initial stages of the ε-PL production phase led to the accumulation of amino acids generated from pyruvate and l-aspartic 4-semialdehyde, while the ε-PL production was terminated. These findings suggest that de novo biosynthesized nascent l-lysine from the DAP pathway contributes to ε-PL production.
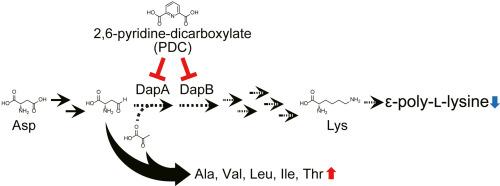
白链霉菌在生产ε-聚赖氨酸过程中,赖氨酸的来源依赖于二氨基磺酸途径。
白球链霉菌(Streptomyces albulus) NBRC14147产生聚阳离子单聚氨基酸ε-聚赖氨酸(ε-PL)。由于其抗菌性能、对人体无毒、可生物降解性和渗透性,对ε-PL的需求量很大。由于ε-PL是由l-赖氨酸聚合产生的,因此确定生产ε-PL所需的l-赖氨酸来源对提高其产量至关重要。在放线菌中,赖氨酸是通过二氨基乙酸(DAP)途径产生的。本研究通过比较结核分枝杆菌DapB的结构和白葡萄球菌DapB的模型结构,确定了2,6-吡啶二羧酸盐(PDC)是DAP途径酶DapB的抑制剂。结果表明,PDC的添加抑制了白球藻的生长。更重要的是,在ε-PL产生的初始阶段,PDC的添加导致丙酮酸和l-天冬氨酸4-半醛生成的氨基酸积累,而ε-PL的产生被终止。这些发现表明,通过DAP途径从头合成的新生赖氨酸有助于ε-PL的产生。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of bioscience and bioengineering
生物-生物工程与应用微生物
CiteScore
5.90
自引率
3.60%
发文量
144
审稿时长
51 days
期刊介绍:
The Journal of Bioscience and Bioengineering is a research journal publishing original full-length research papers, reviews, and Letters to the Editor. The Journal is devoted to the advancement and dissemination of knowledge concerning fermentation technology, biochemical engineering, food technology and microbiology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


