Effects of 4-month treatment with glycocalyx dietary supplement on endothelial glycocalyx and vascular function after COVID-19 infection
Abstract
Background
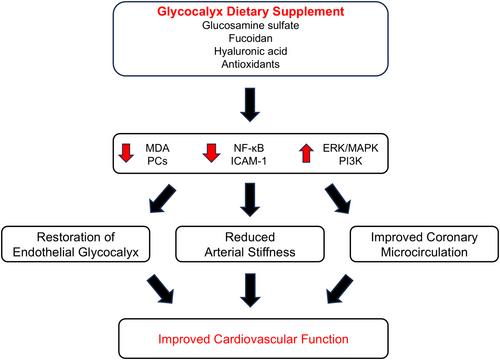
Coronavirus disease 2019 (COVID-19) has been associated with impaired endothelial and vascular function. We investigated whether intervention with glycocalyx dietary supplement (GDS), containing glucosamine sulfate and fucoidan, improves endothelial glycocalyx and vascular function after COVID-19 infection.
Methods
Fifty-seven convalescent patients 14 days after mild-to-moderate COVID-19 infection managed in an outpatient setting were randomized to receive GDS (n = 29) or placebo (n = 28) for 4 consecutive months. We measured at baseline and at 4 months: (a) perfused boundary region (PBR) of the sublingual microvessels with a diameter range of 4–25 μm, as a marker of endothelial glycocalyx integrity, (b) pulse wave velocity and augmentation index, (c) coronary flow reserve using Doppler echocardiography, and (d) malondialdehyde and protein carbonyls as oxidative stress markers.
Results
Four months after treatment, patients who received GDS showed a greater reduction in PBR 4–25 μm (−6.8% vs. −1.3%), pulse wave velocity (−13.2% vs. −3%), augmentation index (−28.5% vs. −2.5%), malondialdehyde (−26% vs. −2.9%), protein carbonyls (−31.3% vs. −1%) and a greater increase in coronary flow reserve (12.9% vs. 1.6%) compared to placebo (p < .05). In the GDS group, the reduction in PBR 4–25 μm was associated with the corresponding decrease in pulse wave velocity (r = .31, p = .047), malondialdehyde, and protein carbonyls, as well as with the increase in coronary flow reserve (r = −.59, p = .008) at follow-up. Post-treatment, none of the patients under GDS reported post-COVID symptoms compared to 21.4% of the patients under placebo.
Conclusion
Four-month treatment with GDS may improve endothelial glycocalyx and vascular function after COVID-19 infection.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT05185934.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


