Regulation of MCCC1 expression by a Parkinson’s disease-associated intronic variant: implications for pathogenesis
IF 2.5
3区 生物学
Q2 GENETICS & HEREDITY
引用次数: 0
Abstract
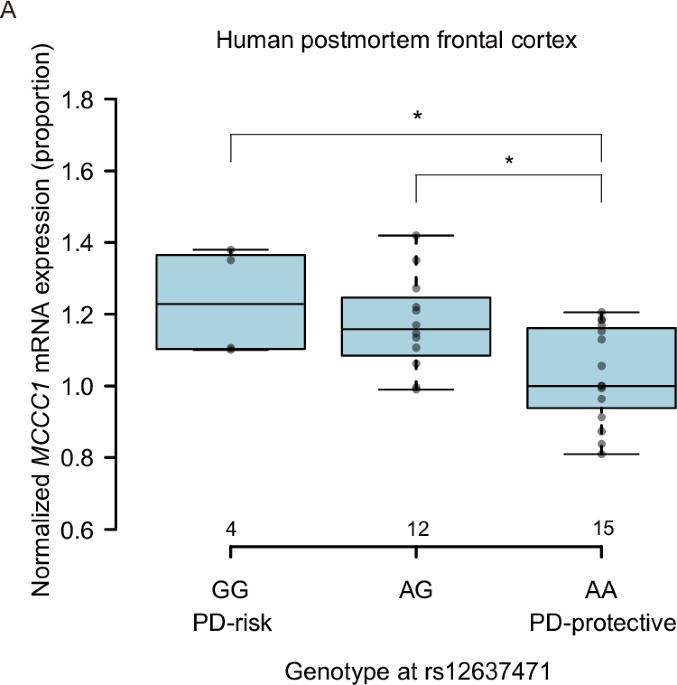
Parkinson’s disease (PD) is a common neurodegenerative disorder characterized by dopaminergic neuron loss and α-synuclein aggregation. While some familial cases result from single-gene mutations, most are sporadic, involving complex genetic and environmental interactions. Among PD risk loci identified through genome-wide association studies, MCCC1 encodes a mitochondrial enzyme essential for leucine catabolism; however, the causal variant remains unclear. Here, we investigated whether the intronic variant rs12637471 regulates MCCC1 mRNA expression and influences PD risk. Postmortem brain analysis revealed significantly elevated MCCC1 mRNA levels in G-allele carriers, consistent with peripheral tissue eQTL data from GTEx. Using CRISPR/Cas9-edited induced pluripotent stem cells, we generated isogenic lines differing only at rs12637471 and observed increased MCCC1 expression in G-allele dopaminergic neurons. Given MCCC1’s mitochondrial role, its dysregulation may impact mitochondrial homeostasis, autophagy, or inflammation, potentially contributing to PD pathogenesis.
帕金森病相关内含子变异对MCCC1表达的调控:对发病机制的影响
帕金森病(PD)是一种常见的神经退行性疾病,以多巴胺能神经元丧失和α-突触核蛋白聚集为特征。虽然一些家族性病例是由单基因突变引起的,但大多数是散发的,涉及复杂的遗传和环境相互作用。在通过全基因组关联研究确定的帕金森病风险位点中,MCCC1编码一种对亮氨酸分解代谢至关重要的线粒体酶;然而,因果变量仍不清楚。在这里,我们研究了内含子变异rs12637471是否调节MCCC1 mRNA表达并影响PD风险。死后大脑分析显示,g等位基因携带者的MCCC1 mRNA水平显著升高,与GTEx的外周组织eQTL数据一致。使用CRISPR/ cas9编辑的诱导多能干细胞,我们产生了仅在rs12637471位点不同的等基因系,并观察到g等位基因多巴胺能神经元中MCCC1表达增加。鉴于MCCC1在线粒体中的作用,其失调可能影响线粒体稳态、自噬或炎症,从而可能导致帕金森病的发病。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Human Genetics
生物-遗传学
CiteScore
7.20
自引率
0.00%
发文量
101
审稿时长
4-8 weeks
期刊介绍:
The Journal of Human Genetics is an international journal publishing articles on human genetics, including medical genetics and human genome analysis. It covers all aspects of human genetics, including molecular genetics, clinical genetics, behavioral genetics, immunogenetics, pharmacogenomics, population genetics, functional genomics, epigenetics, genetic counseling and gene therapy.
Articles on the following areas are especially welcome: genetic factors of monogenic and complex disorders, genome-wide association studies, genetic epidemiology, cancer genetics, personal genomics, genotype-phenotype relationships and genome diversity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


