Accelerated C-F bond cleavage on sulfur vacancy-contained CoS2/MoS2 electrode featuring rich H*
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Carbon-fluorine (C-F) toxic groups in halogenated contaminants pose long-standing and significant threat to aquatic ecosystems and human health. In tackling the challenge of breaking C-F bond with low energy-consuming technique, this study reveals an enhanced electrochemical dehalogenation (ECHD) process using three-dimensional multilayer CoS2/MoS2 heterojunction cathode which possesses sulfur vacancies (Sv)-assisted modulation. It is found out that, when CoS2 and MoS2 grow together, Sv sites are constructed between MoS2 and CoS2 layers, fundamentally reversing the planar electronic inertness of MoS2 and enhancing the coupling between H+ and reactive electrons, thereby generating substantial atomic hydrogen (H*). Meanwhile, the electronic interactions between Sv and neighboring S (Sn) enhance the adsorption of H* at Sn sites and suppressing the competitive H2 evolution, constructing an H*-rich network. Accordingly, H* induces the substantial dechlorination of florfenicol (FLO), and the released free chloride ions indirectly optimized the C-F bond cleavage energy barrier, facilitating the cleavage of C-F bonds. With Sv-assisted modulated ECHD system and taking florfenicol as a representative, we accomplish near-total C-F bond removal with 100 % selectivity at -1.4 VAg/AgCl, marking a leap beyond current capabilities in efficiency, selectivity, environmental sustainability, and stability over 240-minute waste-free cycle.
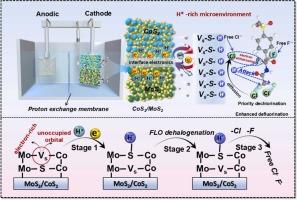
富H*的含硫空穴CoS2/MoS2电极加速C-F键裂解
卤化污染物中的碳氟(C-F)有毒基团对水生生态系统和人类健康构成长期和重大威胁。为了解决以低能耗技术打破C-F键的挑战,本研究揭示了一种使用具有硫空位(Sv)辅助调制的三维多层CoS2/MoS2异质结阴极的增强电化学脱卤(ECHD)工艺。研究发现,当CoS2和MoS2一起生长时,在MoS2和CoS2层之间构建了Sv位,从根本上逆转了MoS2的平面电子惰性,增强了H+与反应电子之间的耦合,从而产生了大量的原子氢(H*)。同时,Sv与相邻的S (Sn)之间的电子相互作用增强了H*在Sn位点的吸附,抑制了H2的竞争性演化,构建了一个富H*网络。因此,H*诱导氟苯尼考(FLO)的大量脱氯,释放的游离氯离子间接优化了C-F键的裂解能垒,促进了C-F键的裂解。利用sv辅助的调节ECHD系统,并以氟苯尼col为代表,我们以-1.4 VAg/AgCl的速度,以100% %的选择性完成了几乎全部的C-F键去除,标志着在240分钟的无废物循环中,在效率、选择性、环境可持续性和稳定性方面超越了目前的能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


