1H, 13C and 15N resonance assignments for the acetyltransferase domain of the kinetoplastid kinetochore protein KKT23 from Trypanosoma brucei
IF 0.6
4区 生物学
Q4 BIOPHYSICS
引用次数: 0
Abstract
KKT23 is a kinetoplastid-specific kinetochore protein that has a C-terminal GCN5-related histone acetyltransferase domain that acetylates the C-terminal tail of histone H2A. Here, we present the 1H, 13C and 15N resonance assignments for the C-terminal region of KKT23 (KKT23125–348) from Trypanosoma brucei in complex with known cofactors for acetyltransferases, acetyl coenzyme A and coenzyme A. These assignments provide the starting point for detailed investigation of the structure, dynamics and interactions of KKT23 in solution.
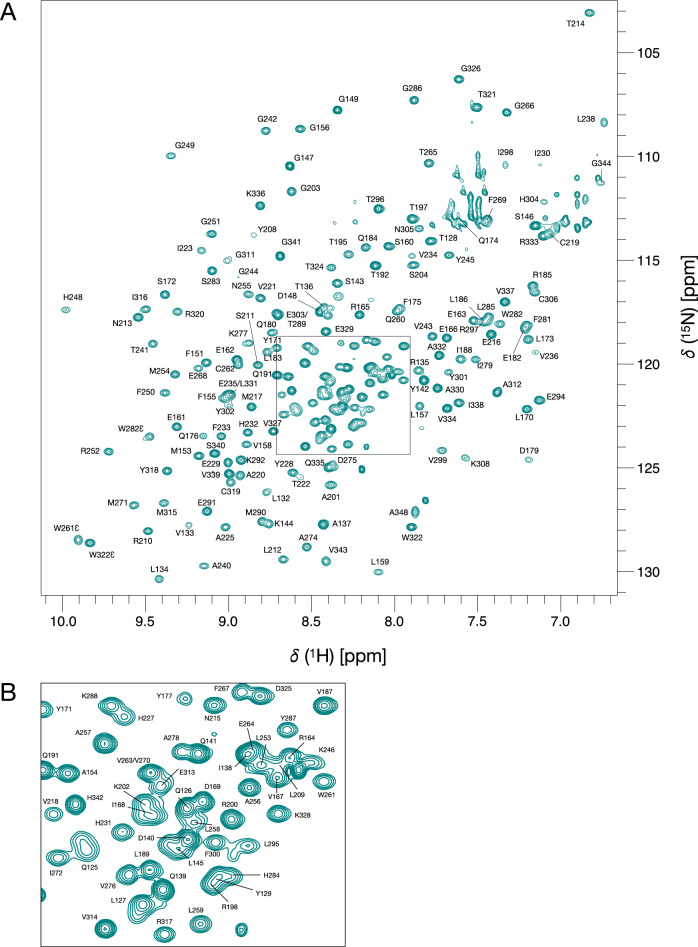
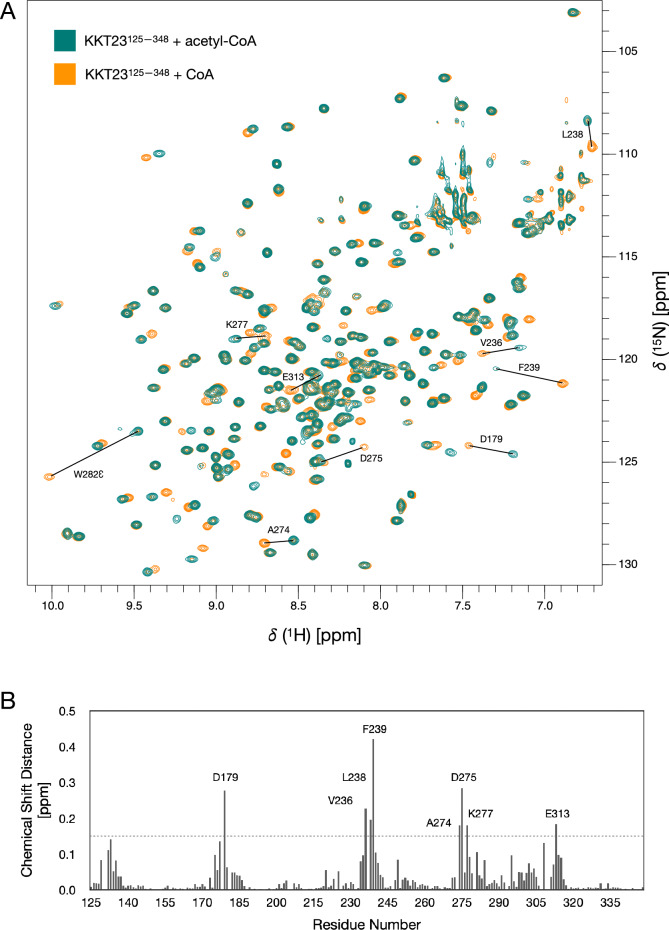
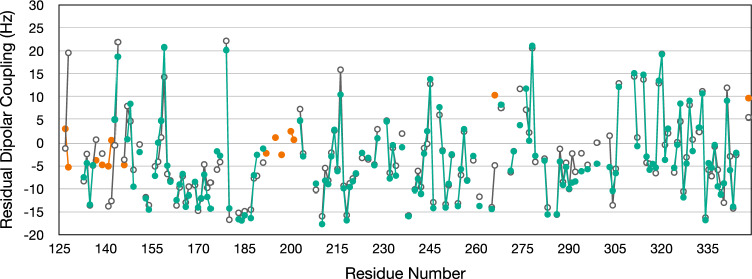
布鲁氏锥虫着丝粒蛋白KKT23乙酰转移酶结构域的1H, 13C和15N共振分配。
KKT23是一种着丝质体特异性的着丝粒蛋白,具有c端gcn5相关的组蛋白乙酰转移酶结构域,可使组蛋白H2A的c端尾部乙酰化。在这里,我们给出了来自布氏锥虫的KKT23 (KKT23125-348)的c端区域与已知的乙酰转移酶、乙酰辅酶A和辅酶A的辅因子的1H、13C和15N共振赋值,这些赋值为详细研究KKT23在溶液中的结构、动力学和相互作用提供了起点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomolecular NMR Assignments
生物-光谱学
CiteScore
1.70
自引率
11.10%
发文量
59
审稿时长
6-12 weeks
期刊介绍:
Biomolecular NMR Assignments provides a forum for publishing sequence-specific resonance assignments for proteins and nucleic acids as Assignment Notes. Chemical shifts for NMR-active nuclei in macromolecules contain detailed information on molecular conformation and properties.
Publication of resonance assignments in Biomolecular NMR Assignments ensures that these data are deposited into a public database at BioMagResBank (BMRB; http://www.bmrb.wisc.edu/), where they are available to other researchers. Coverage includes proteins and nucleic acids; Assignment Notes are processed for rapid online publication and are published in biannual online editions in June and December.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


