Demonstration of the effective intestinal immunity activity of a high branched rhamnogalacturonan-I type pectin polysaccharide from wolfberry via exploration its interaction with mechanical barrier
IF 12.5
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Rhamnogalacturonan-I type pectin polysaccharide from wolfberry has immunity activity, but its intestinal immunity activity and structure-activity relationship in the small intestine was still unclear. This study comparatively investigated the intestinal immune activity of wolfberry-derived high and low branched Rhamnogalacturonan-I type pectin polysaccharides (H-LBP and L-LBP) and explored the interaction mechanism with mechanical barrier. In the normal mechanical barrier model, both H-LBP and L-LBP could cross mechanical barrier with transport rates of 23.2 % and 25 %, thereby directly enhancing macrophage viability and phagocytic ability after crossing the mechanical barrier. The transport mechanism of H-LBP in mechanical barrier included the clathrin- and caveolin-mediated pathways. In the damaged mechanical barrier model, H-LBP could significantly enhance mechanical barrier integrity, reduce the production of neurotransmitter (NO) and inflammatory cytokine (TNF-α), thereby exerting indirectly intestinal immune activity. Transcriptome analysis showed that the interaction mechanism between H-LBP and damaged mechanical barrier mainly involved signaling pathway regulating cell growth and survival (PI3K-AKT). Western blot experiment and molecular docking simulation confirmed that H-LBP could reduce the expression of p-PI3K, p-AKT and cleaved Caspase3. H-LBP had stronger directly and indirectly intestinal immune activity than L-LBP. These findings were useful for the application of H-LBP in improving intestinal immunity oral formulations.
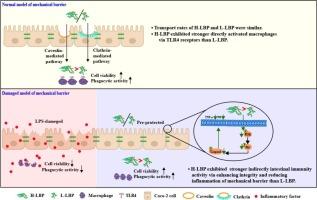
枸杞中高支链鼠李糖半乳葡聚糖- 1型果胶多糖与机械屏障的相互作用证明其有效的肠道免疫活性
枸杞鼠李糖半乳葡聚糖- i型果胶多糖具有免疫活性,但其肠道免疫活性及其在小肠中的构效关系尚不清楚。本研究对比研究了枸杞源性高支鼠李糖半乳葡聚糖- i型果胶多糖(H-LBP和L-LBP)的肠道免疫活性,并探讨了其与机械屏障的相互作用机制。在正常机械屏障模型中,H-LBP和L-LBP均能穿越机械屏障,转运率分别为23.2%和25%,从而直接增强巨噬细胞穿越机械屏障后的活力和吞噬能力。H-LBP在机械屏障中的转运机制包括网格蛋白介导的途径和小窝蛋白介导的途径。在机械屏障受损模型中,H-LBP可显著增强机械屏障的完整性,减少神经递质(NO)和炎性细胞因子(TNF-α)的产生,从而间接发挥肠道免疫活性。转录组分析显示,H-LBP与受损机械屏障的相互作用机制主要涉及调节细胞生长和存活的信号通路(PI3K-AKT)。Western blot实验和分子对接模拟证实,H-LBP可降低p-PI3K、p-AKT和cleaved Caspase3的表达。H-LBP具有较强的直接和间接肠道免疫活性。本研究结果对H-LBP在改善肠道免疫的口服制剂中的应用具有一定的指导意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbohydrate Polymers
化学-高分子科学
CiteScore
22.40
自引率
8.00%
发文量
1286
审稿时长
47 days
期刊介绍:
Carbohydrate Polymers stands as a prominent journal in the glycoscience field, dedicated to exploring and harnessing the potential of polysaccharides with applications spanning bioenergy, bioplastics, biomaterials, biorefining, chemistry, drug delivery, food, health, nanotechnology, packaging, paper, pharmaceuticals, medicine, oil recovery, textiles, tissue engineering, wood, and various aspects of glycoscience.
The journal emphasizes the central role of well-characterized carbohydrate polymers, highlighting their significance as the primary focus rather than a peripheral topic. Each paper must prominently feature at least one named carbohydrate polymer, evident in both citation and title, with a commitment to innovative research that advances scientific knowledge.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


