Spatiotemporally resolved GPCR interactome uncovers unique mediators of receptor agonism
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Cellular signaling by membrane G protein-coupled receptors (GPCRs) is governed by a complex and diverse array of mechanisms. The dynamics of a GPCR interactome, as it evolves over time and space in response to an agonist, provide a unique perspective on pleiotropic signaling decoding and functional selectivity at the cellular level. In this study, we utilized proximity-based APEX2 proteomics to investigate the interaction network of the luteinizing hormone receptor (LHR) on a minute-to-minute timescale. We developed an analytical approach that integrates quantitative multiplexed proteomics with temporal reference profiles, creating a platform to identify the proteomic environment of APEX2-tagged LHR at the nanometer scale. LHR activity is finely regulated spatially, leading to the identification of putative interactors, including the Ras-related GTPase RAP2B, which modulate both receptor signaling and post-endocytic trafficking. This work provides a valuable resource for spatiotemporal nanodomain mapping of LHR interactors across subcellular compartments.
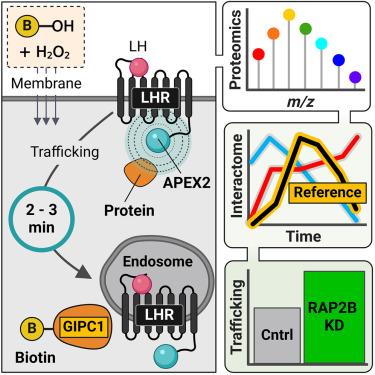
时空分辨的GPCR相互作用揭示了受体激动作用的独特介质
细胞膜G蛋白偶联受体(gpcr)的细胞信号传导是由一系列复杂多样的机制控制的。GPCR相互作用组响应激动剂随时间和空间的演变,为细胞水平上的多效性信号解码和功能选择性提供了独特的视角。在这项研究中,我们利用基于接近度的APEX2蛋白质组学研究了黄体生成素受体(LHR)在分钟到分钟时间尺度上的相互作用网络。我们开发了一种将定量多路蛋白质组学与时间参考谱相结合的分析方法,创建了一个在纳米尺度上识别apex2标记的LHR蛋白质组学环境的平台。LHR活性在空间上受到精细调节,从而确定了可能的相互作用因子,包括ras相关的GTPase RAP2B,它可以调节受体信号传导和内吞后转运。这项工作为跨亚细胞区室的LHR相互作用的时空纳米域映射提供了宝贵的资源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


