Quantification of oleanolic acid and apigenin in biological matrices following intraperitoneal administration of co-loaded nanoformulations: Method validation and biodistribution studies
IF 3.7
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
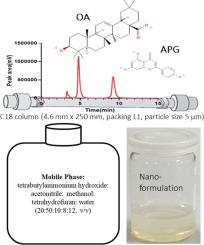
Oleanolic acid (OA) and apigenin (APG) demonstrate a wide range of pharmacological effects, including antioxidant, anti-inflammatory, antiviral, antidiabetic, hepatoprotective, and cardioprotective activities. Both compounds show anticancer potential and have been used individually to treat various cancer types, creating a rationale for their combined use. However, quantifying both drugs in quality and plasma samples presents challenges due to differences in their physicochemical properties and absorption wavelengths (OA: 210–215 nm, APG: 212, 269, and 337 nm). We developed and validated a novel High-Performance Liquid Chromatography UV detection (HPLC-UV) method to address this problem. Using a C18 column (4.6 mm x 250 mm, packing L1, 5 µm particle size) and a mobile phase of acetonitrile: methanol: tetrahydrofuran: water (60:20:8:12, v/v) with a detection wavelength of 215 nm. The method achieved retention times of 4.7 and 10 minutes for OA and APG in quality samples, and 4.9 and 9.8 minutes in plasma samples, respectively. The plasma sample was prepared by simple protein precipitation step. All validation parameters, including sensitivity, selectivity, precision, accuracy, robustness, and stability, met the required criteria. The validated HPLC-UV bioanalytical method was effectively applied in quantification, stability, and pharmacokinetic studies of co-loaded nanoformulations of OA and APG with particle size 163 nm. The entrapment efficiency for OA was 93.95 ± 3.50 %, and for APG, it was 94.5 ± 2.31 % in combined nanoformulation, establishing it as a promising analytical technique for detecting these anticancer agents in complex matrices such as blood, plasma, and polymeric systems.
腹腔注射共载纳米制剂后生物基质中齐墩果酸和芹菜素的定量:方法验证和生物分布研究
齐墩果酸(OA)和芹菜素(APG)具有广泛的药理作用,包括抗氧化、抗炎、抗病毒、抗糖尿病、肝保护和心脏保护活性。这两种化合物都显示出抗癌潜力,并已被单独用于治疗各种类型的癌症,这为它们的联合使用创造了一个基本原理。然而,由于其物理化学性质和吸收波长(OA: 210-215 nm, APG: 212、269和337 nm)的差异,定量药物质量和血浆样品都存在挑战。我们开发并验证了一种新的高效液相色谱紫外检测(HPLC-UV)方法来解决这个问题。采用C18色谱柱(4.6 mm × 250 mm,填料L1,粒径5µm),流动相为乙腈:甲醇:四氢呋喃:水(60:20:8:12,v/v),检测波长为215 nm。该方法在优质样品中OA和APG的保留时间分别为4.7和10分钟,在血浆样品中分别为4.9和9.8分钟。血浆样品采用简单的蛋白质沉淀法制备。所有验证参数,包括灵敏度、选择性、精密度、准确度、稳健性和稳定性,均满足要求标准。建立的HPLC-UV生物分析方法可有效地用于粒径为163 nm的OA和APG共载纳米制剂的定量、稳定性和药动学研究。在联合纳米制剂中,OA的包封效率为93.95±3.50%,APG的包封效率为94.5±2.31%,这为检测血液、血浆和聚合物等复杂基质中的抗癌药物奠定了良好的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


