Novel Al(OH)3 and Ti/Fe@Al(OH)3 nano catalyzed of N-(3-((E)-3-(4-Adamant-1-yl)-phenyl) acryloyl) phenyl) quinoline-2-carboxamide Synthesis and its Molecular Docking, Quantum chemical Studies
IF 4.3
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
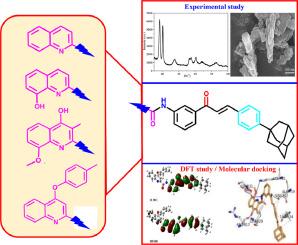
Using a green chemical Al(OH)3 and Ti/Fe@Al(OH)3 nanocatalyzed method, a number of new adamantyl substituted quinoline based chalcone derivative 1 to chalcone derivative 4 (CD1 to CD4) have been synthesized. The synthesized CD1 to CD4 compounds were examined using a range of spectroscopic methods, such as mass spectrometry, elemental analysis, ¹H![]() NMR, ¹³C
NMR, ¹³C![]() NMR, and FT-IR. These chalcone derivatives (CD1 to CD4) had significant docking scores of -8.06 kcal/mol and -8.94 kcal/mol towards EGFR receptor with more binding affinity, primarily targeting the quinazoline inhibitor (PDB ID: 1M17, Lapitinib), according to in silico molecular docking studies. Measurements using fluorescence and UV–Vis spectroscopy also revealed significant variations in the emission and absorption spectra. Compounds CD1 to CD4 HOMO and LUMO values were found using cyclic voltammetry (CV) investigations, which also showed the charge transfer properties between intramolecular atoms (D-π-A). A maximal red shift was seen in the emission spectra at 500 nm, and this value increased with solvent polarity. Current study green chemical synthesis method utilized for synthesizing novel therapeutic anti-cancer chalcone derivatives for clinical pertinence.
NMR, and FT-IR. These chalcone derivatives (CD1 to CD4) had significant docking scores of -8.06 kcal/mol and -8.94 kcal/mol towards EGFR receptor with more binding affinity, primarily targeting the quinazoline inhibitor (PDB ID: 1M17, Lapitinib), according to in silico molecular docking studies. Measurements using fluorescence and UV–Vis spectroscopy also revealed significant variations in the emission and absorption spectra. Compounds CD1 to CD4 HOMO and LUMO values were found using cyclic voltammetry (CV) investigations, which also showed the charge transfer properties between intramolecular atoms (D-π-A). A maximal red shift was seen in the emission spectra at 500 nm, and this value increased with solvent polarity. Current study green chemical synthesis method utilized for synthesizing novel therapeutic anti-cancer chalcone derivatives for clinical pertinence.
新型Al(OH)3和Ti/Fe@Al(OH)3纳米催化N-(3-((E)-3-(4-Adamant-1-yl)-苯基)丙烯酰)苯基)喹啉-2-羧酰胺合成及其分子对接、量子化学研究
采用绿色化学Al(OH)3和Ti/Fe@Al(OH)3纳米催化的方法,合成了一些新的金刚烷基取代喹啉基查尔酮衍生物1到查尔酮衍生物4 (CD1到CD4)。利用质谱、元素分析、¹HNMR、¹³CNMR和FT-IR等多种光谱方法对合成的CD1至CD4化合物进行检测。根据硅分子对接研究,这些查尔酮衍生物(CD1到CD4)与EGFR受体的对接评分分别为-8.06 kcal/mol和-8.94 kcal/mol,具有更强的结合亲和力,主要针对喹唑啉抑制剂(PDB ID: 1M17,拉匹替尼)。使用荧光和紫外-可见光谱的测量也揭示了发射和吸收光谱的显著变化。利用循环伏安法(CV)测定了化合物CD1到CD4的HOMO和LUMO值,并测定了分子内原子间的电荷转移性质(D-π-A)。发射光谱在500 nm处出现最大红移,且随溶剂极性的增加而增大。本研究利用绿色化学合成方法合成具有临床针对性的新型抗癌查尔酮衍生物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics Impact
Materials Science-Materials Science (miscellaneous)
CiteScore
2.60
自引率
0.00%
发文量
65
审稿时长
46 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


