Novel gas-diffusion microextraction followed by gas chromatography coupled to tandem mass spectrometry methodology for the determination of fragrance allergens in cosmetic products
IF 6.5
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
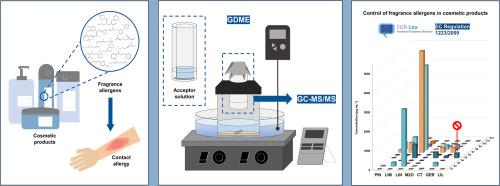
An efficient sample preparation method using gas-diffusion microextraction (GDME) followed by gas chromatography coupled to tandem mass spectrometry (GC-MS/MS) is proposed for the first time to determine fragrance allergens in both aqueous and alcohol-based cosmetic products. The most significant GDME parameters were optimized, starting with extraction temperature as a preliminary experiment. Subsequently, an experimental design was performed to evaluate the influence of six parameters: acceptor solution volume, acetonitrile percentage in the acceptor solution, sample dilution, salting-out effect, extraction time, and sample volume. Under the optimized conditions, the method was validated in terms of linearity, precision, trueness, obtaining a good performance. The validated methodology was applied to twelve real cosmetic samples, demonstrating the widespread occurrence of these allergens in cosmetics. Notably, lilial, a compound prohibited by Regulation EC No 1223/2009, was detected in one cosmetic product (460 μg mL-1); and the concentrations of some of the target fragrance allergens in some samples reach values above 1000 μg mL-1. This methodology represents a sustainable and practical approach, supported by AGREEPrep and BAGI metrics, respectively.
新型气相扩散微萃取-气相色谱-串联质谱法测定化妆品中香氛过敏原
首次建立了气相扩散微萃取(GDME) -气相色谱-串联质谱(GC-MS/MS)联用的高效样品制备方法,用于测定水基和醇基化妆品中的香氛过敏原。从提取温度作为初步实验入手,优化了最显著的GDME参数。随后,进行了实验设计,以评估六个参数的影响:受体溶液体积、受体溶液中乙腈的百分比、样品稀释度、盐析效果、提取时间和样品体积。在优化条件下,对该方法进行了线性度、精密度、准确度等方面的验证,取得了较好的效果。经验证的方法应用于12个真实化妆品样品,表明这些过敏原在化妆品中广泛存在。值得注意的是,在一种化妆品中检测到460 μg mL-1的丁香,这是欧盟法规1223/2009禁止的化合物;部分样品中目标香氛过敏原的浓度达到1000 μg mL-1以上。该方法代表了一种可持续和实用的方法,分别得到AGREEPrep和BAGI指标的支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


