A highly efficient tungsten carbide catalyst for natural Photothermal-Driven formic acid dehydration reaction
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
While the dehydration of formic acid (FA) to produce high-purity carbon monoxide (CO) is theoretically promising, practical application has been hindered by the lack of cost-effective catalysts with high selectivity and stability. In this study, using a straightforward method, we synthesize tungsten carbide (WC) nanoparticles uniformly distributed on porous foam carbon (WC@PFC). This catalyst can achieve a high CO production rate of 342.0 mmol g−1h−1 with 99.8 % selectivity at 250 °C, demonstrating its great potential for FA dehydration. In-situ diffuse reflectance infrared Fourier transform spectroscopy confirms the only reaction pathway for FA molecules to form CO and H2O on WC@PFC through carboxyl (COOH*) intermediates. Density functional theory (DFT) calculations confirm that the COOH* intermediate is more likely to form CO/H2O rather than CO2/H2, as the energy barrier for dehydration is lower than that for dehydrogenation. In addition, we utilize a custom-made TiC/Cu-based solar heating device to achieve a high temperature of 253 °C under a weak solar-irradiation intensity of 0.5 kW m−2, which is enough for the thermal catalytic FA dehydration required. With the assistance of the solar heating device, the catalyst shows exceptional performance in photothermal catalytic experiments, maintaining CO selectivity near 100 %. The outstanding activity, high selectivity, and long-term stability of WC@PFC establish it as a promising catalyst for producing high-purity CO through FA dehydration under sunlight irradiation without external energy input.
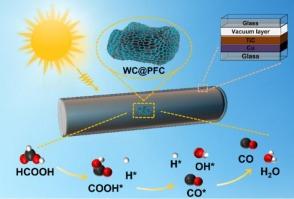
天然光热驱动甲酸脱水反应的高效碳化钨催化剂
甲酸(FA)脱水制备高纯度一氧化碳(CO)在理论上是有希望的,但由于缺乏高选择性和高稳定性的高成本效益催化剂,阻碍了其实际应用。在这项研究中,我们使用一种简单的方法,合成了均匀分布在多孔泡沫碳(WC@PFC)上的碳化钨(WC)纳米颗粒。在250℃条件下,该催化剂的CO产率可达342.0 mmol g−1h−1,选择性为99.8%,显示了其在FA脱水中的巨大潜力。原位漫反射红外傅里叶变换光谱证实了FA分子通过羧基(COOH*)中间体在WC@PFC上生成CO和H2O的唯一反应途径。密度泛函理论(DFT)计算证实COOH*中间体更容易形成CO/H2O而不是CO2/H2,因为脱水的能垒低于脱氢的能垒。此外,我们利用定制的TiC/ cu基太阳能加热装置,在0.5 kW m−2的弱太阳辐照强度下实现了253°C的高温,足以满足热催化FA脱水所需的温度。在太阳能加热装置的辅助下,催化剂在光热催化实验中表现出优异的性能,保持了接近100%的CO选择性。WC@PFC具有优异的活性、高选择性和长期稳定性,是一种很有前途的催化剂,可用于在日光照射下无外部能量输入的FA脱水制备高纯度CO。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


