Can Labs Help With Vaccination? In Vitro Tests in Diagnosis of Allergy to COVID-19 Vaccines–A Systematic Review
Abstract
Introduction
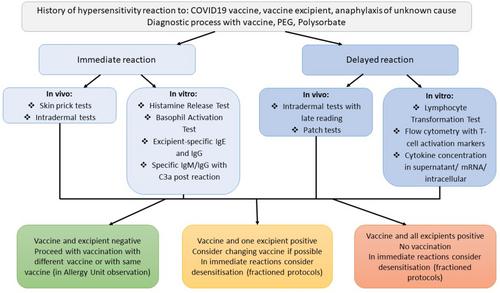
Since the outbreak of the coronavirus pandemic in 2019, vaccinations have proven to be a key strategy in disease prophylaxis. Although vaccines are safe from the perspective of the general population, hypersensitivity reactions have still been described, causing individuals to be reluctant in their vaccination decision. Since the description of first reports of COVID-19 vaccine allergy, many protocols of allergy work-up have been developed, including In Vitro and In Vivo tests. Although In Vivo tests were more accessible, many patients preferred In Vitro tests that would not involve contact with the allergen and be safe. This applied in particular to patients that had experienced a severe delayed hypersensitivity reaction in which In Vivo tests were highly limited and provocations were deemed high risk. Taking into account these circumstances, In Vitro tests might significantly enhance allergy work-up.
Methods
National Center for Biotechnology Information (Pubmed) database was searched in May 2024 for articles on In Vitro diagnostic methods for COVID-19 vaccine allergy and hypersensitivity.
Results
This article describes the In Vitro tests developed to date in the diagnosis of COVID-19 vaccine hypersensitivity: (1) analysis of specific IgE and IgG, (2) Basophil Activation Test, (3) Histamine Release Test, (4) IgM-dependent complement activation, (5) Lymphocyte Transformation Test, (6) Flow cytometry T-Cell markers, (7) Th1/Th2 cytokines concentration in cell culture.
Conclusions
The article highlights the tests' advantages, flaws and possible clinical applications.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


