A pH-responsive production of hydroxyl radical in Fenton process
IF 14.3
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Efficient management of temporal latency and spatial heterogeneity remains a critical challenge in sensor-based pH regulation for smart water management, primarily due to inherent response delays and mass transfer constraints. In oxidation systems with dynamic pH environments, delayed responses can lead to issues such as cyanide release, unwanted side reactions, or pipe damage. To address these challenges, we propose a “pause-then-adjust” control strategy, exploiting the pH-responsive generation of hydroxyl radicals (•OH) in a modified Fenton reaction system. This system utilizes hydroxylamine as an electron donor and ethylenediaminetetraacetic acid (EDTA) as a stabilizer for iron ions. Within the pH range of 7.0–10.0, the coexistence of [Fe2+-EDTA]2− and [Fe3+-OH-EDTA]2− complexes facilitates efficient electron transfer, resulting in the selective and sustained production of •OH radicals. The inherent pH-responsiveness of this strategy enables rapid and spatially coherent adjustments, offering a robust supplementary method for addressing complex and evolving requirements in advanced water treatment systems.
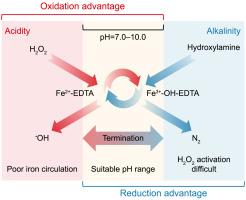
Fenton过程中羟基自由基对ph值的响应
有效管理时间延迟和空间异质性仍然是基于传感器的智能水管理pH调节的关键挑战,主要是由于固有的响应延迟和传质限制。在具有动态pH环境的氧化系统中,延迟反应可能导致氰化物释放、不良副反应或管道损坏等问题。为了解决这些挑战,我们提出了一种“暂停然后调整”的控制策略,利用改进的芬顿反应体系中羟基自由基(•OH)的ph响应生成。该系统利用羟胺作为电子供体,乙二胺四乙酸(EDTA)作为铁离子的稳定剂。在7.0 ~ 10.0的pH范围内,[Fe2+-EDTA]2−和[Fe3+-OH-EDTA]2−配合物的共存有利于有效的电子转移,从而选择性地持续产生•OH自由基。该策略固有的ph响应性使快速和空间一致的调整成为可能,为解决高级水处理系统中复杂和不断变化的要求提供了一种强大的补充方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Science and Ecotechnology
Multiple-
CiteScore
20.40
自引率
6.30%
发文量
11
审稿时长
18 days
期刊介绍:
Environmental Science & Ecotechnology (ESE) is an international, open-access journal publishing original research in environmental science, engineering, ecotechnology, and related fields. Authors publishing in ESE can immediately, permanently, and freely share their work. They have license options and retain copyright. Published by Elsevier, ESE is co-organized by the Chinese Society for Environmental Sciences, Harbin Institute of Technology, and the Chinese Research Academy of Environmental Sciences, under the supervision of the China Association for Science and Technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


