Electrochemical assay for the quantification of anticancer drugs and their inhibition mechanism
IF 4.3
3区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
Overexpression of pyruvate kinase (PyK) is linked to many kinds of malignant tumors, representing therefore one of the most promising therapeutic targets for cancer treatment. Inhibition of PyK slows down tumor growth or causes tumor cell death, minimizing cancer cell proliferation, and understanding inhibitor mechanism of action can significantly improve cancer therapy. The present work describes the use of an amperometric bienzymatic biosensor, based on PyK and pyruvate oxidase (PyOx), in enzyme inhibition studies of four kinase inhibitors, CPG77675, Nilotinib, Ruxolitinib, Cerdulatinib. Their inhibition mechanism is studied and discussed in detail, with a thorough evaluation of their enzyme-inhibitor complex binding constants (Ki) and the inhibitor concentration required for 50% inhibition (IC50), employing standard inhibition procedure graphical methods. The biosensor is successfully applied for the quantification of the inhibitors by fixed potential amperometry, with excellent detection limit values in the pM range. It is the first detection method reported for the anticancer drugs CPG77675 and Cerdulatinib. The electrochemical assay based on the biosensor brings several advantages over the available assay kits for high-throughput screening (HTS) of kinase inhibitors, namely: low cost, easy operability and robustness demonstrated by biosensor high reproducibility and both operational and storage stability, offering an opportunity to discover new inhibitors and optimize their therapeutic index.
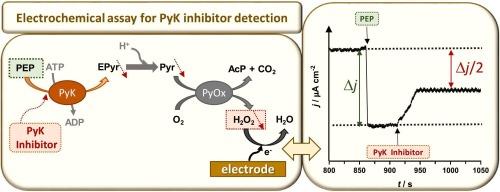
电化学法定量测定抗癌药物及其抑制机制
丙酮酸激酶(pyruvate kinase, PyK)的过表达与多种恶性肿瘤有关,因此是癌症治疗中最有希望的治疗靶点之一。抑制PyK可减缓肿瘤生长或导致肿瘤细胞死亡,使癌细胞增殖最小化,了解抑制剂的作用机制可显著改善癌症治疗。本研究描述了一种基于PyK和丙酮酸氧化酶(PyOx)的电流型双酶生物传感器在四种激酶抑制剂CPG77675、尼罗替尼、鲁索替尼、Cerdulatinib的酶抑制研究中的应用。对其抑制机制进行了详细的研究和讨论,并采用标准抑制程序图解法,全面评估了它们的酶-抑制剂复合物结合常数(Ki)和50%抑制所需的抑制剂浓度(IC50)。该生物传感器成功地应用于固定电位安培法对抑制剂的定量,在pM范围内具有良好的检测限。这是首次报道的针对抗癌药物CPG77675和Cerdulatinib的检测方法。与现有的激酶抑制剂高通量筛选(HTS)分析试剂盒相比,基于生物传感器的电化学分析具有以下几个优点:成本低、易操作、生物传感器显示的鲁棒性、高重复性、操作和存储稳定性,为发现新的抑制剂和优化其治疗指数提供了机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Methods
生物-生化研究方法
CiteScore
9.80
自引率
2.10%
发文量
222
审稿时长
11.3 weeks
期刊介绍:
Methods focuses on rapidly developing techniques in the experimental biological and medical sciences.
Each topical issue, organized by a guest editor who is an expert in the area covered, consists solely of invited quality articles by specialist authors, many of them reviews. Issues are devoted to specific technical approaches with emphasis on clear detailed descriptions of protocols that allow them to be reproduced easily. The background information provided enables researchers to understand the principles underlying the methods; other helpful sections include comparisons of alternative methods giving the advantages and disadvantages of particular methods, guidance on avoiding potential pitfalls, and suggestions for troubleshooting.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


