Overcoming Conformational Complexity to Elucidate Selective Ethylene Tetramerization Behavior
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The famous metallacycle mechanism for ethylene tetramerization has received serious attention in recent decades, as the possibility of further ring expansion may not ensure the specific formation of 1-octene. However, there are limited discussions available on a precise understanding of the ethylene tetramerization behavior. Herein, a detailed density functional theory investigation was performed to explore continuous metallacyclic chain growth. Based on the well-defined Cr/PNP complex, computational results demonstrated that the flexibility of metallacycles plays a key role in controlling the ring expansion. A careful conformational search revealed that prior to the next ethylene insertion, the highly flexible nine-membered ring can rapidly eliminate to 1-octene via the 3,9-H shift transition state, which adopts a unique boat-chair form to afford minimal nonbonded repulsion, while a geometric constraint resulting from the β-H agostic interaction balances the ethylene migratory insertion step, which diminished the impact of ring flexibility. As a result, the selective formation of 1-octene can be expected, while the production of higher oligomer or even high molecular-weight polyethylene via the extended metallacycle pathway is less likely to occur. The expansion of conformationally flexible metallacycles may promote the H-elimination step, which indicated that continuous metallacyclic chain growth is hindered in operation. This study not only contributes to a better understanding of the diverse modes regarding ethylene conversion but also highlights the impact of conformational complexity on mechanistic studies.
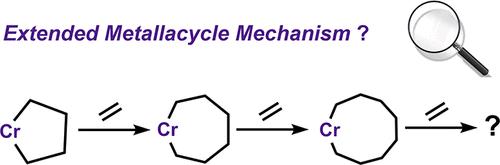
克服构象复杂性阐明选择性乙烯四聚行为
近几十年来,著名的乙烯四聚化金属环机制受到了人们的重视,因为进一步扩环的可能性可能无法保证1-辛烯的特异性形成。然而,关于乙烯四聚行为的精确理解的讨论有限。本文对金属环链的连续生长进行了详细的密度泛函理论研究。基于定义良好的Cr/PNP配合物,计算结果表明,金属环的柔韧性对控制环的膨胀起着关键作用。仔细的构象搜索表明,在下一次乙烯插入之前,高柔性的九元环可以通过3,9- h移位过渡态快速消除到1-辛烯,该过渡态采用独特的船椅形式,以提供最小的非键排斥,而由β-H相互作用产生的几何约束平衡了乙烯迁移插入步骤,这减少了环灵活性的影响。因此,1-辛烯的选择性形成是可以预期的,而通过延伸的金属循环途径生产高低聚物甚至高分子量聚乙烯的可能性较小。构象柔性金属环的扩展可能会促进h消除步骤,这表明在操作过程中,金属环链的连续生长受到阻碍。该研究不仅有助于更好地理解乙烯转化的各种模式,而且突出了构象复杂性对机理研究的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


