Dual-site doping of PET-derived carbon quantum dots with alkali-earth and transition metals for adsorption of PFOS, ibuprofen, sucralose, and decabromodiphenyl oxide
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Emerging toxic pollutants (EPs) such as perfluorooctane sulfonic acid (PFOS), ibuprofen (IBU), sucralose, and decabromodiphenyl oxide (BDE-209) pose significant threats to environmental and human health due to their persistence and bioaccumulation. This study investigates the efficacy of polyethylene terephthalate (PET)-derived carbon quantum dots (CQDs) functionalized with dual-site doping of alkali-earth (Ca, Mg) and transition (Zn, Fe) metals at graphitic and carbonyl (C=O) sites for the adsorption of these EPs. Using computational modeling and density functional theory (DFT), we analyzed the structural, electronic, and adsorption properties of pristine and metal-doped PET-CQDs. Results reveal that metal doping enhances surface area, solvent accessibility, and electronic reactivity, with Ca-O and Mg-O doping yielding the highest Connolly surface areas (299.54 Å2 and 289.11 Å2) and Fe-G reducing the HOMO-LUMO gap to 1.40 eV, improving charge transfer. Adsorption studies indicate that Fe-doped CQDs exhibit the strongest binding energies for PFOS (−5264.3 kcal/mol) and IBU (−8209.88 kcal/mol), driven by electrostatic and hydrogen bonding interactions, while BDE-209 shows the highest adsorption energy (−23335 kcal/mol) across all CQDs due to π-π stacking and hydrophobic effects. Sucralose displays weaker adsorption, with positive binding energies indicating limited affinity. Post-adsorption molecular dynamics highlight increased mobility in Ca-O and Zn-G CQDs, with diffusivity constants rising significantly (e.g., 0.03482 for IBU on Ca-O), while Fe-G and Mg-O CQDs show rigidity, reflecting stronger pollutant retention. Proposed mechanisms involve ion–dipole, electrostatic, hydrogen bonding, and π-π stacking interactions tailored by metal type and doping site. These findings elucidate structure–property relationships, demonstrating that dual-site metal doping enhances the selectivity and efficiency of PET-CQDs, offering a sustainable approach for designing advanced adsorbents for water treatment applications.

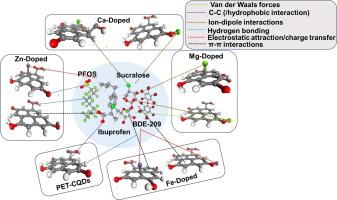
碱土和过渡金属掺杂pet衍生碳量子点对全氟辛烷磺酸、布洛芬、三氯蔗糖和十溴二苯氧基氧化物的吸附
新出现的有毒污染物(EPs),如全氟辛烷磺酸(PFOS)、布洛芬(IBU)、三氯氯和十溴二苯氧化物(BDE-209),由于其持久性和生物蓄积性,对环境和人类健康构成重大威胁。本研究考察了聚对苯二甲酸乙二醇酯(PET)衍生的碳量子点(CQDs)在石墨和羰基(C=O)位点上双位置掺杂碱土(Ca, Mg)和过渡金属(Zn, Fe)对EPs的吸附效果。利用计算模型和密度泛函理论(DFT)分析了原始PET-CQDs和掺杂金属PET-CQDs的结构、电子和吸附特性。结果表明,金属掺杂提高了比表面积、溶剂可及性和电子反应性,其中Ca-O和Mg-O掺杂产生了最高的Connolly表面积(299.54 Å2和289.11 Å2), Fe-G将HOMO-LUMO间隙减小到1.40 eV,促进了电荷转移。吸附研究表明,在静电和氢键作用的驱动下,fe掺杂的CQDs对PFOS(- 5264.3 kcal/mol)和IBU(- 8209.88 kcal/mol)的结合能最强,而BDE-209由于π-π堆叠和疏水效应,在所有CQDs中表现出最高的吸附能(- 23335 kcal/mol)。三氯蔗糖的吸附能力较弱,其结合能为正,表明亲和力有限。吸附后的分子动力学表明,Ca-O和Zn-G CQDs中的迁移率增加,扩散常数显著上升(例如,IBU在Ca-O上的扩散常数为0.03482),而Fe-G和Mg-O CQDs表现出刚性,反映出更强的污染物滞留。提出的机制包括离子偶极子,静电,氢键和π-π堆叠相互作用,根据金属类型和掺杂位置定制。这些发现阐明了结构-性能关系,表明双金属掺杂提高了PET-CQDs的选择性和效率,为设计用于水处理应用的高级吸附剂提供了可持续的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


