Rhenium Bronze Oxide Containing Tellurium Ions with Two Lone Pairs
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Hexagonal bronze oxides are represented by the chemical formula AxMO3, where A is an electropositive element such as alkali metal or alkaline earth metal and M is a transition metal such as Mo, W, or Re. We report the first synthesis of a tellurium-containing rhenium bronze oxide, Te0.30ReO3. The compound has an orthorhombic crystal structure, with tellurium located within the ab plane along with rhenium and oxygen, and planarly coordinated to three oxygen atoms. Electron localization function (ELF) analysis revealed that tellurium has two ELF attractors above and below the TeO3 plane and that the ELF structure around tellurium is similar to that of ClF3. Based on valence shell electron pair repulsion theory, two equatorial positions in the coordination structure of the trigonal bipyramid are occupied by lone pairs and the remaining three positions are occupied by oxygen. Electron density calculations demonstrated that the unique coordination structure of the tellurium arises from the hybridization of the 5s and 5p orbitals of tellurium with the 2p orbitals of oxygen.
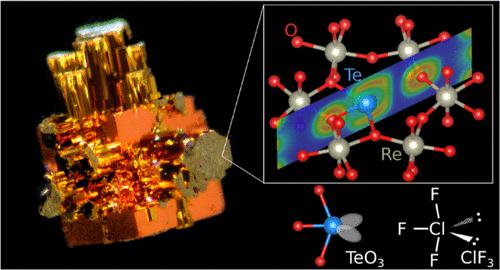
含两孤对碲离子的氧化铼青铜
六方青铜氧化物由化学式AxMO3表示,其中A是正电元素,如碱金属或碱土金属,M是过渡金属,如Mo、W或Re。我们首次合成了含碲铼氧化青铜Te0.30ReO3。该化合物具有正交晶体结构,碲、铼和氧位于ab平面内,并与三个氧原子平面配位。电子局域函数(ELF)分析表明,碲在TeO3平面上下有两个极低频吸引子,其周围的极低频吸引子结构与ClF3相似。根据价壳层电子对斥力理论,三角双锥体配位结构中的两个赤道位置被孤电子对占据,其余三个位置被氧占据。电子密度计算表明,碲独特的配位结构是由于碲的5s和5p轨道与氧的2p轨道杂化所致。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


