Tunable CO2 capture in n-ethylethylenediamine functionalized Mg2-MOF-74: unraveling the role of diamine basicity in reactivity and adsorption capacity
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
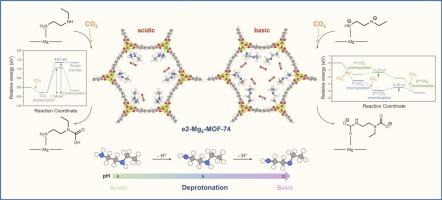
Mitigating CO2 emissions requires the development of highly efficient, tunable, and selective adsorption materials. In this study, we explore the potential of diamine functionalized Mg2-MOF-74 for CO2 capture using density functional theory (DFT) calculations.N-Ethylethylenediamine (e2) exhibits favorable CO2 adsorption energy and a low energy barrier for the rate-determining step (RDS) in the capture process, making it an effective diamine for MOF functionalization. Additionally, this study examines the influence of e2 basicity (pKa) on adsorption mechanisms, reaction barriers, and overall CO2 uptake capacity. Our results reveal that while the neutral e2 exhibits a high RDS energy barrier, its mono- and doubly deprotonated forms significantly lower these barriers. In particular, the energy barriers for CO2 insertion at both amine sites in the doubly deprotonated system are significantly lower (0.23–0.40 eV). We find that the doubly deprotonated e2/Mg2-MOF-74 framework enhances reactivity at both amine sites, enabling the efficient capture of up to 24 CO2 molecules. The moderate adsorption energy of −0.98 eV at high CO2 coverage suggests facile desorption, promoting efficient MOF regeneration. Furthermore, our analysis indicates that a solvent environment with a pH of ∼12 favors the formation of the doubly deprotonated e2 species, further optimizing CO2 adsorption performance
n-乙二胺功能化Mg2-MOF-74中可调节的CO2捕获:揭示了二胺碱度在反应性和吸附能力中的作用
减少二氧化碳排放需要开发高效、可调和选择性吸附材料。在这项研究中,我们利用密度泛函理论(DFT)计算探索了二胺功能化Mg2-MOF-74在二氧化碳捕获方面的潜力。n -乙基乙二胺(e2)具有良好的CO2吸附能和捕获过程中速率决定步骤(RDS)的低能垒,使其成为MOF功能化的有效二胺。此外,本研究还考察了e2碱度(pKa)对吸附机制、反应屏障和总体CO2吸收能力的影响。我们的研究结果表明,虽然中性的e2具有较高的RDS能垒,但其单质子和双质子去质子形式显著降低了这些能垒。特别是,在双去质子化体系中,CO2在两个胺位点插入的能垒明显较低(0.23-0.40 eV)。我们发现双去质子化的e2/Mg2-MOF-74框架增强了两个胺位点的反应性,能够有效捕获多达24个CO2分子。在高CO2覆盖下,中等吸附能为- 0.98 eV,表明脱附容易,促进了MOF的高效再生。此外,我们的分析表明,pH为~ 12的溶剂环境有利于双去质子e2物质的形成,进一步优化了CO2吸附性能
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


