Electrochemical CO2 Reduction to Ethanol: Comparison of Cell Designs, Process Modeling, Downstream Processes, and Techno-Economic Assessments
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The electrochemical carbon dioxide reduction reaction (CO2RR) is of increasing importance for the development of a closed carbon cycle. Ethanol is an attractive target product due to its high energy density and large market size. Therefore, publications describing the CO2RR to ethanol are first compared. Both flow cells and zero-gap cells show the most promising results. However, this work has shown that accumulation of liquid organic products in the catholyte of a flow cell would lead to a significant increase in the overpotential. The resulting low concentration of products in the catholyte (<3.5 mol %), which should not be exceeded, leads to high separation costs. Consequently, a downstream process is developed based on the product stream of the most promising CO2RR in a zero-gap cell. Afterward, a techno-economic assessment (TEA) of the entire process from CO2 capture to CO2RR to product purification was performed. The electricity for the CO2RR is still the main cost driver, followed by the CO2 recovery, whose costs are caused by the low single-pass CO2 conversion. Strategies for improving the entire process are outlined, and an Excel calculation tool for adapting the TEA is provided.
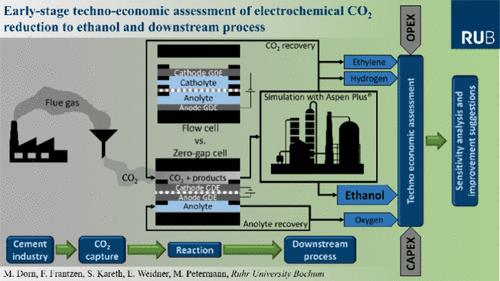
电化学二氧化碳还原为乙醇:电池设计、过程建模、下游过程和技术经济评估的比较
电化学二氧化碳还原反应(CO2RR)对封闭碳循环的发展具有越来越重要的意义。乙醇因其高能量密度和巨大的市场规模而成为极具吸引力的目标产品。因此,首先比较描述CO2RR到乙醇的出版物。流动电池和零间隙电池都显示出最有希望的结果。然而,这项工作表明,液体有机产物在流动电池的阴极液中的积累会导致过电位的显著增加。阴极液中产物的浓度很低(3.5 mol %),不能超过这个浓度,导致分离成本很高。因此,下游工艺是基于零间隙电池中最有前途的CO2RR的产品流而开发的。随后,对从CO2捕集到CO2RR再到产品纯化的整个过程进行了技术经济评估(TEA)。CO2RR的电力仍然是主要的成本驱动因素,其次是二氧化碳回收,其成本是由低单次二氧化碳转换引起的。概述了改进整个过程的策略,并提供了适应TEA的Excel计算工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


