Promoting activation of N2 and H2O via dual active sites synergistically for efficient photocatalytic ammonia synthesis
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Photocatalytic nitrogen fixation is a green synthetic process for the direct conversion of N2 and H2O to ammonia using solar energy. However, the scarcity of N2 and H2O active sites is a limiting factor in the kinetics of nitrogen fixation reactions. The construction of spatially synergistic dual active sites is a feasible strategy to overcome this challenge. Herein, Fe-doped CdS (FCS) photocatalysts containing S vacancy have been prepared by a simple one-step solvothermal method. The results show that S vacancy in FCS preferentially adsorb H2O molecules and activate them to active hydrogen (H*) for nitrogen reduction. Fe sites tend to adsorb N2 molecules and promote the d− band center of CdS to move to Fermi level (Ef), which significantly improves the activation ability of N2 molecules. The unique dual active sites greatly reduce Gibbs free energy of N2 and H2O activation and improve surface reaction kinetics of nitrogen reduction reaction. NH4+ production rate of FCS reached 13.39 μmol · h−1 without sacrificial reagent, which was 13.2 times higher than that of CdS. This study proves the significance of synergy between active sites for improving reaction efficiency and provides inspiration for the construction of dual active systems in photocatalytic nitrogen reduction.
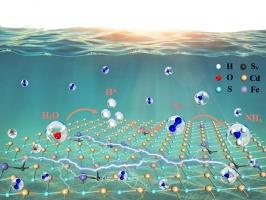

通过双活性位点协同促进N2和H2O的活化,实现高效光催化合成氨
光催化固氮是利用太阳能将N2和H2O直接转化为氨的绿色合成工艺。然而,N2和H2O活性位点的缺乏是固氮反应动力学的限制因素。构建空间协同双活性位点是克服这一挑战的可行策略。本文采用简单的一步溶剂热法制备了含S空位的掺铁CdS (FCS)光催化剂。结果表明,FCS中的S空位优先吸附H2O分子,并将其激活为活性氢(H*)进行氮还原。Fe位倾向于吸附N2分子,促使CdS的d -带中心向费米能级(Ef)移动,显著提高了N2分子的活化能力。独特的双活性位点大大降低了N2和H2O活化的吉布斯自由能,提高了氮还原反应的表面反应动力学。在不牺牲试剂的情况下,FCS的NH4+产率达到13.39 μmol · h−1,是CdS的13.2倍。本研究证明了活性位点之间的协同作用对提高反应效率的重要意义,为光催化氮还原双活性体系的构建提供了启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


