PTPN22 as a therapeutic target in intervertebral disc degeneration: Modulating mitophagy and pyroptosis through the PI3K/AKT/mTOR axis
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Intervertebral disc degeneration (IDD) is a predominant risk factor for low back pain (LBP). However, the mechanisms underlying IDD progression remain unclear.Objectives
The protein tyrosine phosphatase non-receptor type 22 (PTPN22) is associated with various chronic inflammatory and autoimmune conditions. However, its role in the progression of IDD remains obscure. This investigation delves into the function of PTPN22 within IDD and examines its molecular mechanisms.Methods
The expression levels of PTPN22 in human and rat degenerative nucleus pulposus (NP) cells were analyzed using Western blot and immunohistochemistry. Following PTPN22 knockdown via lentiviral transfection, pyroptosis, extracellular matrix (ECM) degradation, mitophagy, and mitochondrial function were assessed using Western blot, immunofluorescence, Calcein-AM/PI staining, qPCR, Seahorse, JC-1, and MitoSOX assays. The roles of autophagy and the PI3K/AKT/mTOR pathway were further investigated using the autophagy inhibitor 3-MA, Baf-A1, and the PI3K agonist 740Y-P. A puncture-induced rat model was established, and the effects of LV-shPTPN22 on IDD were evaluated through imaging and histological analyses.Results
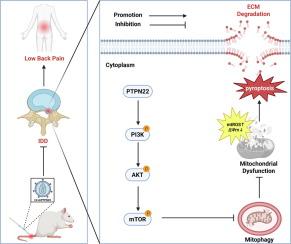
We noted an upregulation of PTPN22 in degenerative NP cells. A deficiency in PTPN22 was found to enhance mitophagy, thereby alleviating hydrogen peroxide (H2O2)-induced mitochondrial dysfunction and consequently mitigating NP cell pyroptosis and ECM degradation. Inhibition of the PI3K/AKT/mTOR pathway appears to play a pivotal role in the protective effects of PTPN22 deficiency against IDD. Experiments conducted in vivo revealed that PTPN22 knockdown significantly curtails the progression of IDD.Conclusion
In summary, PTPN22 knockdown alleviates IDD progression by reducing pyroptosis and ECM degradation through enhanced mitophagy. This highlights PTPN22 as a critical contributor to IDD and a promising therapeutic target.
PTPN22作为椎间盘退变的治疗靶点:通过PI3K/AKT/mTOR轴调节线粒体自噬和焦亡
椎间盘退变(IDD)是腰痛(LBP)的主要危险因素。然而,IDD进展的机制尚不清楚。蛋白酪氨酸磷酸酶非受体22型(PTPN22)与多种慢性炎症和自身免疫性疾病有关。然而,它在IDD进展中的作用仍然不清楚。本研究深入探讨了PTPN22在IDD中的作用,并探讨了其分子机制。方法采用Western blot和免疫组化方法分析PTPN22在人和大鼠退行性髓核(NP)细胞中的表达水平。通过慢病毒转染将PTPN22敲低后,采用Western blot、免疫荧光、Calcein-AM/PI染色、qPCR、Seahorse、JC-1和MitoSOX检测评估焦噬、细胞外基质(ECM)降解、线粒体自噬和线粒体功能。使用自噬抑制剂3-MA、Baf-A1和PI3K激动剂740Y-P进一步研究自噬和PI3K/AKT/mTOR通路的作用。建立针刺诱导大鼠模型,通过影像学和组织学分析评价LV-shPTPN22对IDD的影响。结果我们注意到PTPN22在退行性NP细胞中表达上调。研究发现,缺乏PTPN22可增强线粒体自噬,从而减轻过氧化氢(H2O2)诱导的线粒体功能障碍,从而减轻NP细胞焦亡和ECM降解。抑制PI3K/AKT/mTOR通路似乎在PTPN22缺乏对IDD的保护作用中起关键作用。在体内进行的实验表明,PTPN22的敲除可显著抑制IDD的进展。综上所述,PTPN22基因敲低可通过增强线粒体自噬来减少焦亡和ECM降解,从而减轻IDD的进展。这突出了PTPN22作为IDD的关键贡献者和有希望的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


