Ultrasound-Driven Selenium Nanoparticles Realize Bone Defect Repair through Activating Selenoproteins to Regulate PI3K/AKT Signaling Pathway
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Excessive and variable inflammation in bone defects is a key factor that impedes effective bone repair. Herein, an ultrasound-controlled composite hydrogel (LNT-SeNPs@Gel) integrating gelatin-methacryloyl and lentinan-decorated selenium nanoparticles (LNT-SeNPs) is developed, exhibiting strong antioxidant and anti-inflammatory properties to remodel the inflammatory microenvironment of bone defects. This hydrogel serves as a platform for integrating bifunctional ultrasound (ultrasound modulation, USc and ultrasound for repairing, USr), facilitating cascade treatment and reducing the overall treatment period. During the inflammatory phase of bone repair, USc remotely modulates the LNT-SeNPs@Gel hydrogel, regulating the release of LNT-SeNPs to inhibit the overproduction of reactive oxygen species (ROS) and inflammatory factors, ultimately remodeling the inflammatory microenvironment. Subsequently, USr could activate the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signaling pathway regulated by selenoproteins to enhance the osteogenesis of MC3T3-E1 cells, thereby accelerating the bone repair process. Consequently, the combination of bifunctional ultrasound and LNT-SeNPs@Gel significantly improves bone repair outcomes and reduces the treatment period in rats. In conclusion, this study implies that the coordinated integration of the dual effects of ultrasound is a promising strategy for handling the complex and lengthy bone defects repair.
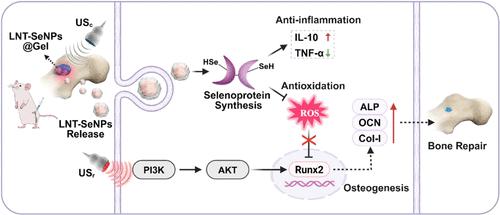
超声驱动硒纳米颗粒通过激活硒蛋白调控PI3K/AKT信号通路实现骨缺损修复
骨缺损中过度和可变的炎症是阻碍有效骨修复的关键因素。本研究开发了一种结合明胶-甲基丙烯酰和香菇酸修饰的硒纳米颗粒(LNT-SeNPs)的超声控制复合水凝胶(LNT-SeNPs@Gel),具有很强的抗氧化和抗炎特性,可以重塑骨缺损的炎症微环境。该水凝胶可作为整合双功能超声(超声调制,USc和超声修复,USr)的平台,促进级联治疗,缩短整体治疗周期。在骨修复的炎症阶段,USc远程调节LNT-SeNPs@Gel水凝胶,调节LNT-SeNPs的释放,抑制活性氧(ROS)和炎症因子的过量产生,最终重塑炎症微环境。随后,USr可激活硒蛋白调控的磷脂酰肌醇3-激酶/蛋白激酶B (PI3K/AKT)信号通路,促进MC3T3-E1细胞成骨,从而加速骨修复过程。因此,双功能超声联合LNT-SeNPs@Gel可显著改善大鼠骨修复效果,缩短治疗周期。综上所述,超声双重作用的协同整合是处理复杂、冗长骨缺损的一种有前景的修复策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


