Electroreduction Mechanism of UO2Cl2 and Nucleation and Morphology of Uranium Dioxide at Different Conditions
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
To investigate the electroreduction mechanism of UO2Cl2 and the nucleation mode and morphology of uranium dioxide, the electrochemical behavior of UO22+ on the tungsten (W) electrode was studied in LiCl–KCl molten salt by cyclic voltammetry. The results demonstrated that UO22+ underwent two sequential single-electron transfers to form UO2. Prior to its reduction to UO2+, UO22+ was initially converted into a low-coordination complex ion. Besides the reduction peak of bulk UO2, a peak associated with the strong adsorption of UO2 on the electrode surface was also observed. The nucleation mode of UO2 was examined by chronoamperometry, revealing that the nucleation mechanism was related to UO2Cl2 concentration. The influence of current densities on nucleation morphology was studied using an atomic force microscope and found the nuclei was smaller and denser at higher current density. The deposits obtained by galvanostatic electrolysis at different current densities were examined by scanning electron microscopy–energy-dispersive X-ray spectroscopy and X-ray diffraction, showing that a regular octahedral UO2 was formed at low current density, while a dendritic UO2 was formed at high current density. A two-step electrolysis with an initial high current density followed by a low current density was conducted, and a dense UO2 with a lychee-like morphology was produced.
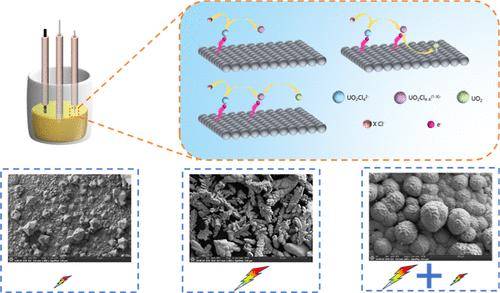
UO2Cl2的电还原机理及不同条件下二氧化铀的成核和形貌
为了研究UO2Cl2的电还原机理以及二氧化铀的成核方式和形态,采用循环伏安法研究了UO22+在LiCl-KCl熔盐中在钨电极上的电化学行为。结果表明,UO22+经过两次连续的单电子转移形成UO2。在还原为UO2+之前,UO22+首先转化为低配位络合物离子。除了大块UO2的还原峰外,还观察到一个与UO2在电极表面的强吸附有关的峰。用时间电流法研究了UO2的成核模式,发现成核机制与UO2Cl2浓度有关。利用原子力显微镜研究了电流密度对成核形貌的影响,发现电流密度越大,原子核越小,密度越大。采用扫描电镜、能谱和x射线衍射对不同电流密度下恒流电解得到的UO2进行了表征,结果表明:低电流密度下形成了正八面体UO2,高电流密度下形成了树枝状UO2。采用初始高电流密度后低电流密度的两步电解工艺,制得具有荔枝样形貌的致密UO2。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


