PLA2G15 is a BMP hydrolase and its targeting ameliorates lysosomal disease
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
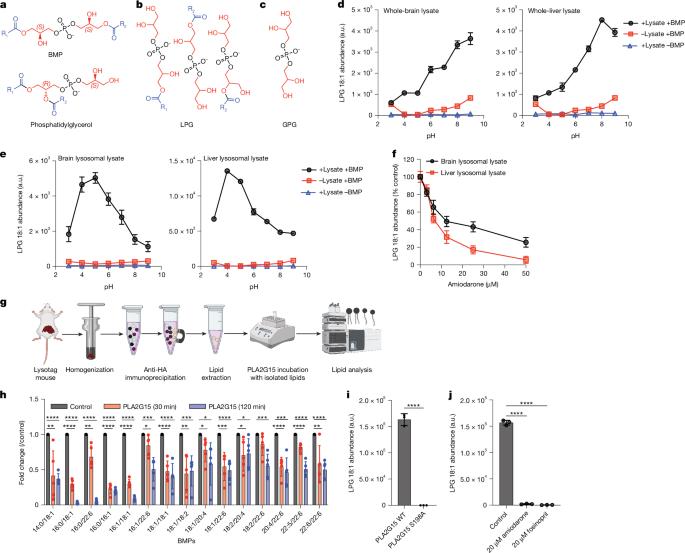
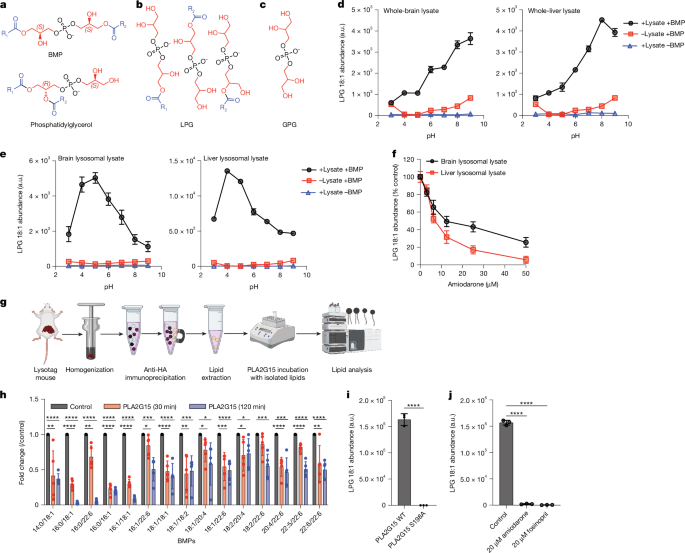
Lysosomes catabolize lipids and other biological molecules, maintaining cellular and organismal homeostasis. Bis(monoacylglycero)phosphate (BMP), a major lipid constituent of intralysosomal vesicles, stimulates lipid-degrading enzymes and is altered in various human conditions, including neurodegenerative diseases1,2. Although lysosomal BMP synthase was recently discovered3, the enzymes mediating BMP turnover remain elusive. Here we show that lysosomal phospholipase PLA2G15 is a physiological BMP hydrolase. We further demonstrate that the resistance of BMP to lysosomal hydrolysis arises from its unique sn2, sn2′ esterification position and stereochemistry, as neither feature alone confers resistance. Purified PLA2G15 catabolizes most BMP species derived from cell and tissue lysosomes. Furthermore, PLA2G15 efficiently hydrolyses synthesized BMP stereoisomers with primary esters, challenging the long-held thought that BMP stereochemistry alone ensures resistance to acid phospholipases. Conversely, BMP with secondary esters and S,S stereoconfiguration is stable in vitro and requires acyl migration for hydrolysis in lysosomes. Consistent with our biochemical data, PLA2G15-deficient cells and tissues accumulate several BMP species, a phenotype reversible by supplementing wild-type PLA2G15 but not its inactive mutant. Targeting PLA2G15 reduces the cholesterol accumulation in fibroblasts of patients with Niemann–Pick disease type C1 and significantly ameliorates disease pathologies in Niemann–Pick disease type C1-deficient mice, leading to an extended lifespan. Our findings established the rules governing BMP stability in lysosomes and identified PLA2G15 as a lysosomal BMP hydrolase and a potential target for therapeutic intervention in neurodegenerative diseases. Lysosomal phospholipase PLA2G15 was identified as a physiological BMP hydrolase whose activity depends on unique esterification and stereochemistry of BMP and offers a potential therapeutic target for Niemann–Pick disease type C1 and other neurodegenerative conditions.
PLA2G15是一种BMP水解酶,其靶向性可改善溶酶体疾病
溶酶体分解脂质和其他生物分子,维持细胞和生物体的稳态。双(单酰基甘油)磷酸(BMP)是溶酶体内囊泡的主要脂质成分,刺激脂质降解酶,并在各种人类疾病中发生改变,包括神经退行性疾病1,2。虽然最近发现了溶酶体BMP合成酶,但介导BMP转换的酶仍然是未知的。我们发现溶酶体磷脂酶PLA2G15是一种生理性BMP水解酶。我们进一步证明BMP对溶酶体水解的抗性源于其独特的sn2, sn2 '酯化位置和立体化学,因为这两个特征都不能单独产生抗性。纯化的PLA2G15可分解代谢大多数来源于细胞和组织溶酶体的BMP。此外,PLA2G15有效地水解合成BMP立体异构体和伯酯,挑战了长期以来认为BMP立体化学本身可以确保对酸性磷脂酶的抗性。相反,具有二级酯和S,S立体构型的BMP在体外是稳定的,需要酰基迁移才能在溶酶体中水解。与我们的生化数据一致,缺乏PLA2G15的细胞和组织积累了几种BMP,这种表型可以通过补充野生型PLA2G15而不是其失活突变体来逆转。靶向PLA2G15可减少尼曼-皮克病C1型患者成纤维细胞中的胆固醇积累,并显著改善尼曼-皮克病C1型缺陷小鼠的疾病病理,从而延长寿命。我们的研究结果建立了溶酶体中BMP稳定性的规则,并确定了PLA2G15作为溶酶体BMP水解酶和神经退行性疾病治疗干预的潜在靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


