Enantioselective Friedel–Crafts Reaction for the Synthesis of 4,7-Difunctionalized Indoles Featuring a Chiral Heteroatom-Substituted Quaternary Carbon at the C7 Position
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Enantioselective functionalization of 4-aminoindoles at the site-specific C7 position via a chiral phosphoric acid-catalyzed Friedel–Crafts reaction with cyclic thioimidates was developed. This approach enables the formation of 4,7-difunctionalized indoles incorporating the chiral N,S-acetal motif with excellent yield and enantioselectivity (up to 99% yield and >99% ee). The protocol is also compatible with isatin-derived ketoimines for the highly enantioselective synthesis of 4,7-difunctionalized indole derivatives bearing aza-quaternary carbon at the C7 position. The synthetic potential was demonstrated by gram-scale experiments and versatile transformations of the products, with the method characterized by low catalyst loading, site specificity, excellent enantioselectivity, and a broad substrate scope.
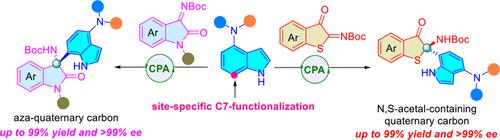
对映选择性Friedel-Crafts反应合成C7位手性杂原子取代季碳的4,7-二官能化吲哚
通过手性磷酸催化的Friedel-Crafts反应,研究了4-氨基吲哚在C7位点的对映选择性功能化。该方法能够形成包含手性N, s -缩醛基序的4,7-二官能化吲哚,具有优异的收率和对映体选择性(高达99%收率和>;99% ee)。该方案也适用于isatin衍生的酮亚胺,用于高度对映选择性合成4,7-二官能化吲哚衍生物,在C7位置上含有氮杂季碳。通过克级实验和产物的多用途转化,证明了该方法的合成潜力,该方法具有催化剂负载低、位点特异性强、对映体选择性好、底物范围广等特点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


