A Radical Approach to Chiral β-Hydroxyboronate Esters via Synergistic Co/Cr Catalysis
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Chiral β-hydroxyboronate esters are versatile intermediates in organic synthesis, serving as bioisosteres of β-hydroxycarboxylic acids and providing access to diverse functionalized molecules. Despite their broad applicability, efficient and general methods for their asymmetric synthesis remain underdeveloped. Here, we report a chromium-catalyzed radical-polar crossover strategy for the asymmetric addition of α-boronate alkyl chlorides to aldehydes, furnishing β-hydroxyboronate esters with a broad substrate scope and high enantioselectivity. The reaction proceeds via CoPc-mediated radical generation followed by Cr-catalyzed stereoselective addition. The resulting products undergo diverse transformations, including oxidation, cross-coupling, and amination, demonstrating their synthetic utility. Mechanistic studies support a monomeric Cr-catalyzed alkylation pathway, expanding the scope of asymmetric Cr catalysis and providing a complementary approach to ionic strategies for β-hydroxyboronate synthesis.
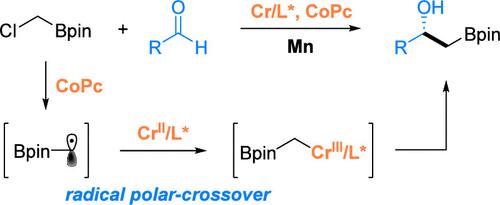
Co/Cr协同催化手性β-羟基硼酸酯的自由基法研究
手性β-羟基硼酸酯是有机合成中的多功能中间体,作为β-羟基羧酸的生物同位体,并提供多种功能化分子的途径。尽管它们具有广泛的适用性,但高效和通用的不对称合成方法尚不发达。在这里,我们报道了一种铬催化的自由基-极性交叉策略,用于α-硼酸烷基氯化物与醛的不对称加成,使β-羟基硼酸酯具有广泛的底物范围和高的对映选择性。反应通过copc介导的自由基生成进行,然后是cr催化的立体选择性加成。所得产物经过多种转化,包括氧化、交叉偶联和胺化,证明了它们的合成效用。机理研究支持单体Cr催化的烷基化途径,扩大了不对称Cr催化的范围,并为离子策略合成β-羟基硼酸盐提供了补充途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


