Evolution process of humins derived from cellulose by a humin extraction approach†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Cellulose utilization has been seriously hindered by the formation of humins, while the humin structure remains challenging since unconverted cellulose and humins exist as a solid mixture. In this work, we developed a novel strategy to extract humins from unconverted cellulose and disclosed the structural evolution process of cellulose-derived humins for the first time. The key intermediate levoglucosan was successfully captured and identified, which significantly favors the formation of anhydro-sugars followed by polymerization due to its stability. By means of comprehensive HPLC-MS/MS, FT-IR, MALDI-TOF and SEM characterization studies, it was proposed that multiple elementary reactions were involved in the formation of cellulose-derived humins, including cellulose depolymerization, etherification, esterification, aldol condensation, dehydration and thermal oxidation. In the early stage, cellulose depolymerization results in glucose and levoglucosan (LG), which undergo etherification to form the early humins via a small molecule mechanism, accompanied by esterification and dehydration. In the later stage, glucooligosaccharides especially with the LG end from cellulose depolymerization undergo etherification via an oligomer mechanism. Meanwhile, etherification of HMF and aldol condensation with LA take place prominently, together with dehydration and oxidation, resulting in the enhancement of CC and CO conjugation.
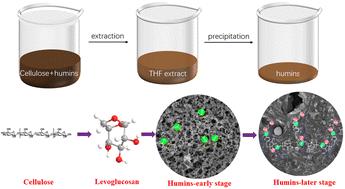
从纤维素中提取人蛋白的进化过程
纤维素的利用严重阻碍了人蛋白的形成,而人蛋白的结构仍然具有挑战性,因为未转化的纤维素和人蛋白以固体混合物的形式存在。在这项工作中,我们开发了一种从未转化的纤维素中提取人蛋白的新策略,并首次揭示了纤维素来源的人蛋白的结构演化过程。成功捕获并鉴定了关键中间体左旋葡聚糖,由于其稳定性,有利于无水糖的形成和聚合。通过HPLC-MS/MS、FT-IR、MALDI-TOF和SEM的综合表征研究,提出纤维素源人源蛋白的形成涉及纤维素解聚、醚化、酯化、醛醇缩合、脱水和热氧化等多种基本反应。在早期阶段,纤维素解聚产生葡萄糖和左旋葡聚糖(LG),通过小分子机制发生醚化反应形成早期人蛋白,并伴有酯化和脱水反应。在后期阶段,低聚糖特别是纤维素解聚的LG端通过低聚物机制进行醚化反应。同时,HMF的醚化反应和醛醇与LA的缩合反应显著,并伴有脱水和氧化反应,导致CC和CO的共轭作用增强。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


