Red-light-driven enantioselective Minisci-type addition to heteroarenes via recyclable semi-heterogeneous catalysis†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Catalytic asymmetric transformations driven by red light, particularly those involving heterogeneous or semi-heterogeneous catalysis, remain unknown. Herein, the enantioselective red-light-catalysed Minisci-type addition of heteroarenes utilizing mesoporous graphitic carbon nitride (mpg-CN) as a recyclable semi-heterogeneous photocatalyst in the presence of chiral phosphoric acid, generating a series of functionalized chiral heteroarenes in high stereoselectivity (up to >19 : 1 dr, >99% ee), is disclosed. Notably, both the photocatalyst and chiral phosphoric acid can be recycled. The heterogeneous nature of mpg-CN enables easy recovery and recycling, demonstrating that no activity and stereoselectivity were lost after ten runs. These photoredox catalyzed reactions can also be conducted under sunlight irradiation and can successfully be applied to large scale synthesis, showing significant potential for industrial application.
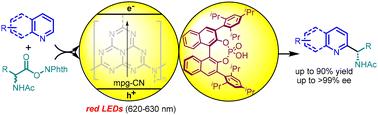
红光驱动的对映选择性minisi型杂环芳烃可回收半多相催化加成反应†
由红光驱动的催化不对称转化,特别是那些涉及多相或半多相催化的,仍然是未知的。本文公开了在手性磷酸存在下,利用介孔石墨氮化碳(mpg-CN)作为可回收的半非均相光催化剂,在红光催化下对映选择性地加成了杂环芳烃,生成了一系列具有高立体选择性(高达19.1 dr, 99% ee)的功能化手性杂环芳烃。值得注意的是,光催化剂和手性磷酸都可以回收。mpg-CN的异构特性使其易于回收和再循环,表明在运行10次后没有活性和立体选择性损失。这些光氧化还原催化反应也可以在阳光照射下进行,并可以成功地应用于大规模合成,具有重要的工业应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


