High Room-Temperature Magnesium Ion Conductivity in Spinel-Type MgYb2Se4 Solid Electrolyte
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
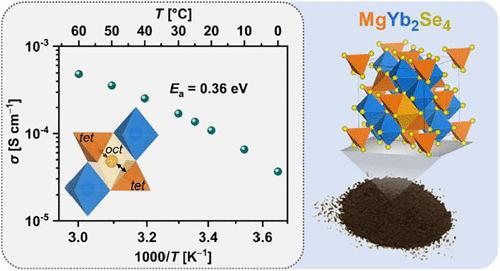
We present three magnesium selenide spinels, MgSc0.4Y0.4Er0.4Tm0.4Yb0.4Se4, Mg0.75Sc2Se3.5Br0.5 and MgYb2Se4, as potential solid electrolytes (SE) for magnesium batteries. In particular, the latter spinel exhibits a room-temperature ionic conductivity exceeding 10–4 S cm–1 and a low Mg2+ migration barrier of 364 meV. The high ionic mobility is attributed to the low magnesium insertion energy revealed by density functional theory (DFT), which indicates a weak binding interaction between magnesium ions and the host lattice. Furthermore, like the two multicomponent spinels, MgYb2Se4 exhibits lower electronic conductivity compared to previously studied MgB2Se4 spinels (B = Sc, Y, Er, Tm) and a good electrochemical stability, making it a strong candidate for Mg2+ SE applications.
尖晶石型MgYb2Se4固体电解质中镁离子的室温高导电性
我们提出了三种硒化镁尖晶石MgSc0.4Y0.4Er0.4Tm0.4Yb0.4Se4, Mg0.75Sc2Se3.5Br0.5和MgYb2Se4作为镁电池的潜在固体电解质。特别是后一种尖晶石的室温离子电导率超过10-4 S cm-1, Mg2+的迁移势垒低至364 meV。高离子迁移率是由于密度泛函理论(DFT)揭示的低镁插入能,这表明镁离子与主晶格之间存在弱结合相互作用。此外,与两种多组分尖晶石一样,与先前研究的MgB2Se4尖晶石(B = Sc, Y, Er, Tm)相比,MgYb2Se4具有更低的电子导电性和良好的电化学稳定性,使其成为Mg2+ SE应用的有力候选材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


