PEBL, a component-based Chinese medicine, reduces virus-induced acute lung injury by targeting FXR to decrease ACE2 levels
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Despite the growing clinical need, the therapeutic efficacy of drugs for acute lung injury (ALI) remains inadequate. Traditional Chinese Medicine (TCM) holds potential in managing ALI due to its unique therapeutic properties. However, the intricate nature of TCM formulations hinders global adoption. Component-based Chinese medicine (CCM) offers a promising pathway for TCM’s internationalization. Phillyrin-Emodin-Baicalin-Liquiritin (PEBL), a CCM with significant anti-inflammatory activity, is derived from the well-established TCM formula Liang-Ge-San. Whether PEBL effectively addresses viral ALI, however, remains unclear.Objectives
This study aims to investigate the therapeutic effects and underlying mechanisms of PEBL on viral ALI.Methods
The efficacy of PEBL against Poly(I:C)-induced ALI was assessed by analyzing cytokine production, macrophage infiltration, pulmonary damage, and mortality. Bioinformatics and network pharmacology were employed to identify key targets and signaling pathways. The molecular mechanisms were further validated using Poly(I:C)-treated RAW264.7 cells, Tg(coro1α: GFP) zebrafish, BALB/c mice, and models of Influenza A/Puerto Rico/8/1934 (H1N1) virus strain (PR8)-induced ALI in BALB/c mice and SARS-CoV-2 Omicron XBB.1.16 subvariant (XBB)-induced ALI in hACE2-transgenic C57BL/6 mice.Results
PEBL mitigated Poly(I:C)-induced ALI, as evidenced by reduced cytokine levels, diminished macrophage infiltration, alleviated lung damage, and decreased mortality. Virtual screening identified the farnesyl X receptor (FXR) and angiotensin-converting enzyme 2 (ACE2) as key therapeutic targets for viral pneumonia. Mechanistically, PEBL downregulated FXR expression, inhibiting FXR binding to ACE2 promoters, which subsequently suppressed NF-κB-p65 nuclear translocation and cytokine production. In vivo, PEBL attenuated cytokine production by inhibiting ACE2 transcription through FXR downregulation, leading to alleviation of Poly(I:C)-induced ALI in both zebrafish and mice. Additionally, PEBL significantly improved symptoms of ALI caused by PR8 and XBB infections, by disrupting the FXR/ACE2 signaling axis, resulting in reduced weight loss, lower lung indices, diminished viral load and titer, fewer pulmonary lesions, and suppressed NF-κB-p65 nuclear translocation, along with decreased cytokine storm.Conclusions
This study provides the first evidence that PEBL offers protective effects against ALI induced by acute respiratory viruses. PEBL prevents FXR from binding to ACE2 by inhibiting FXR transcription, which reduces macrophage infiltration, cytokine storm formation, and inflammatory injury, thereby ameliorating viral ALI. These findings underscore the potential of PEBL as a candidate for further exploration in the treatment of viral ALI.
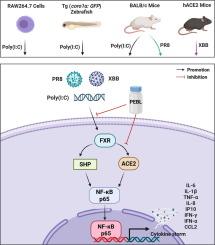
PEBL是一种中药成分,通过靶向FXR降低ACE2水平来减轻病毒诱导的急性肺损伤
尽管临床需求日益增长,但急性肺损伤(ALI)的药物治疗效果仍然不足。传统中医(TCM)由于其独特的治疗特性,在治疗ALI方面具有潜力。然而,中药配方的复杂性阻碍了其在全球的推广。中药组份为中医药国际化提供了一条很有前景的途径。黄芩苷-甘草素(PEBL)是一种具有显著抗炎活性的中药,源自中药复方亮葛散。然而,PEBL是否能有效治疗病毒性ALI仍不清楚。目的探讨PEBL对病毒性ALI的治疗作用及其机制。方法通过分析细胞因子生成、巨噬细胞浸润、肺损伤和死亡率来评价PEBL对Poly(I:C)诱导的ALI的疗效。利用生物信息学和网络药理学来确定关键靶点和信号通路。利用Poly(I:C)处理的RAW264.7细胞、Tg(coro1α: GFP)斑马鱼、BALB/ C小鼠以及甲型流感/波多黎各/8/1934 (H1N1)病毒株(PR8)诱导的BALB/ C小鼠ALI和SARS-CoV-2 Omicron XBB.1.16亚变体(XBB)诱导的hace2转基因C57BL/6小鼠ALI模型,进一步验证了其分子机制。结果spebl减轻Poly(I:C)诱导的ALI,表现为细胞因子水平降低,巨噬细胞浸润减少,肺损伤减轻,死亡率降低。虚拟筛选鉴定出法尼基 X 受体(FXR)和血管紧张素转换酶2 (ACE2)是病毒性肺炎的关键治疗靶点。从机制上讲,PEBL下调FXR表达,抑制FXR与ACE2启动子的结合,从而抑制NF-κB-p65核易位和细胞因子的产生。在体内,PEBL通过FXR下调抑制ACE2转录,从而减轻斑马鱼和小鼠Poly(I:C)诱导的ALI,从而减弱细胞因子的产生。此外,PEBL通过破坏FXR/ACE2信号轴,显著改善PR8和XBB感染引起的ALI症状,导致体重减轻,肺指数降低,病毒载量和滴度降低,肺部病变减少,抑制NF-κB-p65核易位,同时减少细胞因子风暴。结论本研究首次证实PEBL对急性呼吸道病毒引起的ALI具有保护作用。PEBL通过抑制FXR转录阻止FXR与ACE2结合,从而减少巨噬细胞浸润、细胞因子风暴形成和炎症损伤,从而改善病毒性ALI。这些发现强调了PEBL作为进一步探索病毒性ALI治疗候选药物的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


