Application of new approach methodologies for nonclinical safety assessment of drug candidates
IF 101.8
1区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
The development of new approach methodologies (NAMs) and advances with in vitro testing systems have prompted revisions in regulatory guidelines and inspired dedicated in vitro/ex vivo studies for nonclinical safety assessment. This Review by a safety reflection initiative subgroup of the European Federation of Pharmaceutical Industries and Associations (EFPIA)/Preclinical Development Expert Group (PDEG) summarizes the current state and potential application of in vitro studies using human-derived material for safety assessment in drug development. It focuses on case studies from recent projects in which animal models alone proved to be limited or inadequate for safety testing. It further highlights four categories of drug candidates for which alternative in vitro approaches are applicable and discusses progress in using in vitro testing solutions for safety assessment in these categories. Finally, the article highlights new risk assessment strategies, initiatives and consortia promoting the advancement of NAMs. This collective work is meant to encourage the use of NAMs for more human-relevant safety assessment, which should ultimately result in reduced animal testing for drug development. New approach methodologies (NAMs) offer the promise of greater predictivity of potential safety risks of drug candidates, especially for newer types of therapeutics for which animal models alone have limited or no applicability for safety testing. This article identifies four categories of drug candidate for which NAMs are applicable and discusses progress in their use based on case studies from recent projects, as well as initiatives to promote the advancement and use of NAMs for more human-relevant safety assessment.
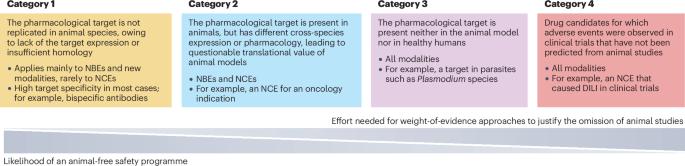

新方法方法在候选药物非临床安全性评估中的应用
新方法方法(NAMs)的发展和体外测试系统的进步促使了监管指南的修订,并激发了用于非临床安全性评估的体外/离体研究。本综述由欧洲制药工业协会联合会(EFPIA)/临床前开发专家组(PDEG)的安全反思倡议小组撰写,总结了在药物开发中使用人源性材料进行安全性评估的体外研究的现状和潜在应用。它侧重于最近项目的案例研究,在这些项目中,单独的动物模型被证明是有限的或不足以进行安全测试。它进一步强调了四类候选药物,其中替代体外方法适用,并讨论了在这些类别中使用体外测试解决方案进行安全性评估的进展。最后,文章重点介绍了新的风险评估策略、倡议和促进NAMs发展的联盟。这项集体工作旨在鼓励使用NAMs进行更多与人类相关的安全性评估,这最终将减少用于药物开发的动物试验。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews. Drug Discovery
医学-生物工程与应用微生物
CiteScore
137.40
自引率
0.30%
发文量
227
期刊介绍:
Nature Reviews Drug Discovery is a monthly journal aimed at everyone working in the drug discovery and development arena.
Each issue includes:
Highest-quality reviews and perspectives covering a broad scope.
News stories investigating the hottest topics in drug discovery.
Timely summaries of key primary research papers.
Concise updates on the latest advances in areas such as new drug approvals, patent law, and emerging industry trends and strategies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


