Glioblastoma Cell Derived Exosomes as a Potent Vaccine Platform Targeting Primary Brain Cancers and Brain Metastases
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Glioblastoma multiforme (GBM) is the most prevalent brain tumor that remains incurable up to now. The rapid advancement of immunotherapy makes vaccines a promising therapeutic approach for GBM. However, current vaccine platforms, such as peptides, dendritic cells, mRNA, and viral vectors, are subject to limitations such as inadequate antigen loading, insufficient immune system activation, ineffective vector delivery, complicated fabrication process, and complex formulation. Here, we developed a GBM tumor cell derived homologous exosomal nanovaccine that does not need to carry any additional tumor antigens and leads to the activation of antigen-presenting cells (APCs) in lymph nodes, increasing the proportion of immune cells (matured dendritic cells, cytotoxic T cells, and memory T cells) and in turn promoting the expression of cytokines (TNF-α, IL-6, and IFN-γ), which effectively stimulates innate immunity to trigger durable protective immunity against tumor cell insult. Our nanovaccine platform possesses efficient dual-targeting capability to lymph nodes and the brain. More importantly, the developed exosomal nanovaccines protected 100% of treated mice by inducing sustained and strong immunity against GL261-luc GBM tumor cells, resulting in 100% mouse survival (8/8) up to 5 months. Our nanovaccines also induced antitumor immune responses in the immunosuppressed CT2A-luc GBM mouse model with greatly improved survival compared to control mice. Exosomal nanovaccines also demonstrated effectiveness in preventing brain metastasis in the B16F10-luc melanoma malignant brain metastasis mouse model, and the mice showed notably improved survival rates. Our simple and potent exosomes offer a versatile platform for clinical translation as individualized vaccine therapy.
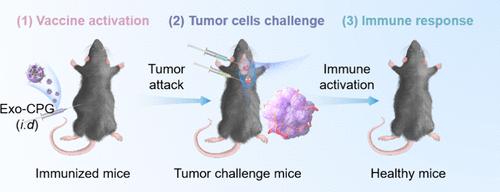
胶质母细胞瘤细胞衍生外泌体作为针对原发性脑癌和脑转移瘤的有效疫苗平台
多形性胶质母细胞瘤(GBM)是目前最常见的无法治愈的脑肿瘤。免疫疗法的快速发展使疫苗成为治疗GBM的一种很有前途的方法。然而,目前的疫苗平台,如多肽、树突状细胞、mRNA和病毒载体,受到诸如抗原装载不足、免疫系统激活不足、载体传递无效、制造过程复杂和配方复杂等限制。在这里,我们开发了一种GBM肿瘤细胞衍生的同源外泌体纳米疫苗,该疫苗不需要携带任何额外的肿瘤抗原,并导致淋巴结中抗原呈递细胞(APCs)的激活,增加免疫细胞(成熟树突状细胞、细胞毒性T细胞和记忆T细胞)的比例,进而促进细胞因子(TNF-α、IL-6和IFN-γ)的表达。有效刺激先天免疫,触发持久的保护性免疫,抵抗肿瘤细胞的侵害。我们的纳米疫苗平台具有对淋巴结和大脑有效的双重靶向能力。更重要的是,所开发的外泌体纳米疫苗通过诱导对GL261-luc GBM肿瘤细胞的持续和强免疫来保护100%的治疗小鼠,使小鼠存活率达到100%(8/8),长达5个月。我们的纳米疫苗还在免疫抑制的CT2A-luc GBM小鼠模型中诱导了抗肿瘤免疫应答,与对照组小鼠相比,存活率大大提高。外泌体纳米疫苗在B16F10-luc黑色素瘤恶性脑转移小鼠模型中也显示出预防脑转移的有效性,并且小鼠的存活率显着提高。我们简单而有效的外泌体为临床翻译提供了一个通用的平台,作为个体化疫苗治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


