Berberine-Functionalized Bismuth-Doped Carbon Dots in a Pathogen-Responsive Hydrogel System: A Multifaceted Approach to Combating Periodontal Diseases
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Periodontal disease, a global health burden linked to dysbiotic oral polymicrobial communities and disrupted immune-inflammatory responses, is critically mediated byPorphyromonas gingivalis(Pg)─the keystone pathogen that sabotages host immunity, triggers tissue inflammation and destruction, and disrupts microbiota balance. Effective therapies should combine antimicrobial action, immune modulation, virulence suppression, and microbiome restoration. Bismuth ions and berberine, which exhibit antimicrobial and epithelial barrier-protecting effects, show potential effectiveness in treating periodontal diseases but face practical limitations due to poor water solubility and bioavailability. To address this, we developed bismuth-doped carbon dots functionalized with structure-modified berberine (BiCD-Ber) as a multifunctional nanomedicine. BiCD-Ber eradicated Pg in various forms, restored Pg-perturbed immune responses in gingival fibroblasts, and preserved epithelial barrier integrity. The doped bismuth ions neutralized Pg virulence factors by blocking the catalytic sites of gingipains. To facilitate in vivo delivery, BiCD-Ber was encapsulated in a disulfide-modified hyaluronic acid hydrogel that degrades in response to Pg metabolites. This BiCD-Ber hydrogel system modulated subgingival microbiota, alleviated inflammation in gingiva, and thereby prevented alveolar bone loss. This approach to concurrently eliminating Pg, modulating inflammatory responses , suppressing virulence factors, and restoring microbiota showcases great potential in managing periodontitis effectively.
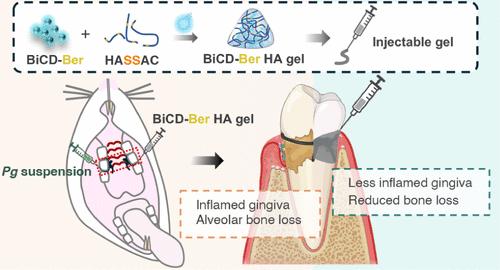
小檗碱功能化铋掺杂碳点在病原体反应水凝胶系统:一个多方面的方法来对抗牙周病
牙周病是一种全球性的健康负担,与口腔多微生物群落失调和免疫炎症反应紊乱有关。牙周病是由牙龈卟啉单胞菌(Pg)介导的,它是破坏宿主免疫力、引发组织炎症和破坏、破坏微生物群平衡的主要病原体。有效的治疗应结合抗菌作用、免疫调节、毒力抑制和微生物群恢复。铋离子和小檗碱具有抗菌和上皮屏障保护作用,在治疗牙周病方面显示出潜在的有效性,但由于水溶性和生物利用度差而面临实际限制。为了解决这个问题,我们开发了以结构修饰小檗碱(BiCD-Ber)功能化的铋掺杂碳点作为多功能纳米药物。bcd - ber根除各种形式的Pg,恢复牙龈成纤维细胞中Pg干扰的免疫反应,并保持上皮屏障的完整性。掺杂铋离子通过阻断牙龈炎的催化位点来中和Pg毒力因子。为了促进体内给药,bcd - ber被包裹在二硫化物修饰的透明质酸水凝胶中,这种水凝胶可以降解Pg代谢物。这种bcd - ber水凝胶系统调节牙龈下微生物群,减轻牙龈炎症,从而防止牙槽骨丢失。这种同时消除Pg、调节炎症反应、抑制毒力因子和恢复微生物群的方法在有效治疗牙周炎方面显示出巨大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


