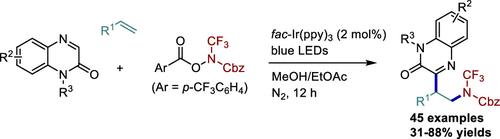
Photoredox-Catalyzed Trifluoromethylamination of Alkenes with Concomitant Introduction of a Quinoxalin-2(1H)-one Moiety
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A photoredox-catalyzed strategy for the difunctionalization of alkenes with quinoxalin-2(1H)-ones and N-CF3 hydroxylamine reagents was developed. This reaction was carried out under photoirradiation conditions, affording the corresponding three-component coupling products in moderate to high yields with excellent regioselectivity. It provides a new protocol to access valuable quinoxalin-2(1H)-one derivatives containing a N-CF3 group.
伴随引入喹啉-2(1H)- 1片段的光氧化还原催化烯烃三氟甲基层化反应
研究了喹啉-2(1H)-酮和N-CF3羟胺光氧化催化烯烃双官能化的方法。该反应在光辐射条件下进行,得到了相应的三组分偶联产物,产率中高,具有优异的区域选择性。它为获得含有N-CF3基团的有价值的喹诺沙林-2(1H)- 1衍生物提供了新的方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


