GSH-Responsive Mn2+ Burst Nanoboxes as Mitophagy Intervention Agents Augment Ferroptosis and Chemoimmunotherapy in Triple-Negative Breast Cancer
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Chemoimmunotherapy is recognized as a promising approach for treating cancer patients. However, the overall therapeutic efficacy of treatments for triple-negative breast cancer (TNBC) is compromised by several factors, including its highly immunosuppressive tumor microenvironment, the presence of drug-resistant cells, and the upregulation of PD-L1 expression induced by chemotherapy. In this study, a GSH-responsive, transferrin-targeted hollow manganese drug-loaded nanobox, employing a strategy to regulate tumor redox balance for integrated MR imaging and treatment, is developed. These nanoboxes enhance chemotherapy sensitivity by activating mitophagy to sensitize ferroptosis and thereby inducing the release of damage-associated molecular patterns (DAMPs) and mitochondrial DNA (mtDNA) from cancer cells. The GSH-responsive release of Mn2⁺, in combination with free DNA, collectively mediates the activation of the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway, thereby ameliorating the immunosuppressive microenvironment. Simultaneously, mtROS-mediated activation of AMP-activated protein kinase (AMPK) overcomes chemotherapy-induced PD-L1 treatment resistance by inducing PD-L1 degradation in tumor cells. Additionally, this process allows for in vitro MR detection of GSH responsiveness and efficient drug delivery. This innovative approach leveraging the interplay of mitophagy and ferroptosis to overcome chemotherapy resistance and immune suppression and enables treatment monitoring.
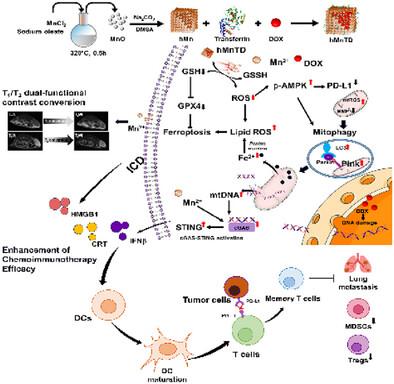
gsh反应性Mn2+爆发纳米盒作为线粒体自噬干预剂增强三阴性乳腺癌的铁凋亡和化学免疫治疗
化学免疫疗法被认为是治疗癌症患者的一种很有前途的方法。然而,三阴性乳腺癌(TNBC)的整体治疗效果受到多种因素的影响,包括其高度免疫抑制的肿瘤微环境、耐药细胞的存在以及化疗诱导的PD-L1表达上调。在这项研究中,研究人员开发了一种gsh响应、转铁蛋白靶向的中空锰药物负载纳米盒,该纳米盒采用一种策略来调节肿瘤氧化还原平衡,以实现MR成像和治疗的一体化。这些纳米盒通过激活线粒体自噬来致敏铁凋亡,从而诱导癌细胞释放损伤相关分子模式(DAMPs)和线粒体DNA (mtDNA),从而增强化疗敏感性。Mn2 +的gsh响应性释放与游离DNA结合,共同介导干扰素基因(STING)通路的环GMP-AMP合成酶(cGAS)刺激因子的激活,从而改善免疫抑制微环境。同时,mtros介导的amp活化蛋白激酶(AMPK)的激活通过诱导肿瘤细胞中的PD-L1降解来克服化疗诱导的PD-L1治疗耐药。此外,该过程允许体外MR检测谷胱甘肽的反应性和有效的药物递送。这种创新的方法利用有丝分裂和铁下垂的相互作用来克服化疗耐药性和免疫抑制,并使治疗监测成为可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


