Modulating the aqueous hydrogen evolution activity of bioinspired diiron electrocatalyst immobilized on carbon nanotubes through various attaching strategy
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Bioinspired chemistry of [FeFe]-hydrogenases with efficient catalytic hydrogen evolution reaction (HER) ability has attracted significant attention in the field of electrochemical water splitting into hydrogen (H2). Herein, this work describes the immobilization of diiron dithiolate (2Fe2S) molecules onto carbon nanotubes (CNTs) through different interfacial attachment and the electrocatalytic HER properties in neutral water. First of all, one all-carbonyl 2Fe2S precursor Fe2(μ-pdt)CO6 [pdt = (SCH2)2CH2, (1)] and its two new PNRP-bridged derivatives Fe2(μ-pdt)CO4(μ-PNRP) [PNRP = (Ph2P)2NR; R = NHCH2pyrene (2) and NHCH2Ph (3)] were prepared and could be further grafted covalently versus non-covalently to CNTs to form three desired 2Fe2S/CNT hybrids labeling as CNT-1 with covalent bond, CNT-2 via π-π stacking, and CNT-3 via physisorption. Meanwhile, the structural characterizations of diiron molecules 2, 3 and CNT-supported hybrids CNT-X (X = 1, 2, 3) are well performed by means of elemental analysis, various spectroscopies, and especially for 2, 3 by X-ray crystallography. Notably, the electrocatalytic HER activity trend of CNT-1 > CNT-2 > CNT-3 is found in 0.1 M phosphate buffer solution (pH = 7) using various electrochemical measurements but the degradation of diiron core in them results in the electrocatalytic deactivations in the long-term electrolysis. Overall, this study is meaningful to extend the practical application of carbon support electrodes integrated with bioinspired diiron electrocatalysts for heterogeneous HER in water.

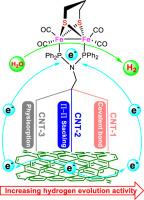
不同附着策略对碳纳米管固定化双铁电催化剂水溶液析氢活性的影响
具有高效催化析氢反应(HER)能力的[FeFe]-加氢酶的生物化学研究在电化学水裂解氢(H2)领域引起了广泛关注。本文描述了二硫代二铁(2Fe2S)分子通过不同的界面附着在碳纳米管(CNTs)上的固定化以及中性水中电催化HER的性能。首先,一个全羰基2Fe2S前体Fe2(μ-pdt)CO6 [pdt = (SCH2)2CH2,(1)]及其两个新的PNRP桥接衍生物Fe2(μ-pdt)CO4(μ-PNRP) [PNRP = (Ph2P)2NR;R = 制备了NHCH2pyrene(2)和NHCH2Ph(3)],并可进一步共价或非共价接枝到CNTs上,形成三种所需的2Fe2S/CNT杂化物,分别标记为共价键CNT-1、π-π堆叠CNT-2和物理吸附CNT-3。同时,通过元素分析、各种光谱,特别是X射线晶体学,对二铁分子2,3和碳纳米管负载的杂化体CNT-X (X = 1,2,3)的结构进行了表征。值得注意的是,CNT-1 >;CNT-2祝辞使用各种电化学测量方法在0.1 M磷酸盐缓冲溶液(pH = 7)中发现了CNT-3,但其中双铁芯的降解导致长期电解中电催化失活性。综上所述,本研究对于拓展碳支撑电极与仿生双铁电催化剂在水中非均相HER中的实际应用具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


