Activating Transition-Metal Oxides through In Situ Regulation of Lower Hubbard Band for Catalytic Conversion of Lithium Polysulfides
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Catalytic conversion of lithium polysulfides (LiPSs) is regarded as an effective avenue to tackle the shuttle effect of lithium–sulfur (Li–S) batteries, especially based upon transition-metal oxides (TMOs). However, the activity origin and corresponding mechanistic insights into such catalytic systems remain elusive. Herein, an activated state associated with the lower Hubbard band (LHB) transition is proposed to elucidate the origin of activity of TMOs by taking Mn3O4 as a model electrocatalyst. Specifically, the broadening of LHB width, the upshift of LHB position, and the orbital rearrangement of LHB, triggered by the in situ substitution of the O atoms in Mn3O4 with the S atoms of LiPSs under working conditions, synergistically enable fast electron transfer and modulate the adsorption capability to a moderate level. Benefiting from these advantages, the Mn3O4 electrocatalyst is converted from the torpid state to the activated state for expediting LiPS conversion. Eventually, the Li–S batteries assembled with Mn3O4 deliver excellent rate performance over 6 C and outstanding cycling stability over 1000 cycles. Moreover, an Ah-scale pouch cell is constructed and delivers a notable energy density of 388.1 W h kg–1. Our work offers a promising pathway based on the regulation of LHB for designing high-performance electrocatalysts for Li–S systems and beyond.
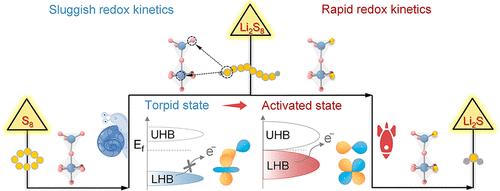
通过原位调节下哈伯德带活化过渡金属氧化物催化转化锂多硫化物
多硫化物锂(LiPSs)的催化转化被认为是解决锂硫电池(li -硫电池)穿梭效应的有效途径,特别是基于过渡金属氧化物(TMOs)。然而,这种催化系统的活性起源和相应的机制见解仍然难以捉摸。本文以Mn3O4为模型电催化剂,提出了一种与下哈伯德带(LHB)跃迁相关的激活态来阐明TMOs活性的来源。具体而言,在工作条件下,由于Mn3O4中的O原子被LiPSs的S原子原位取代,LHB的宽度变宽,LHB位置的上移以及LHB的轨道重排,协同实现了快速的电子转移,并将吸附能力调节到中等水平。利用这些优点,Mn3O4电催化剂从惰性状态转化为活化状态,加速了LiPS的转化。最终,用Mn3O4组装的Li-S电池在6℃以上具有出色的倍率性能,并且在1000次循环以上具有出色的循环稳定性。此外,构建了ah级袋状电池,并提供了388.1 W h kg-1的显著能量密度。我们的工作为设计Li-S系统的高性能电催化剂提供了一条基于LHB调控的有希望的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


