Anchoring Effect-Induced Conformation Remodeling in Epoxy-Functionalized Covalent Organic Frameworks for Enhanced Enzymatic Efficiency
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Covalent organic frameworks (COFs) with tunable structures and versatile chemical functionalities offer great promise for enzyme immobilization. This study explores the “anchoring effect” induced by covalent bonding, which significantly enhances enzymatic efficiency. Glucose oxidase (GOx) was covalently immobilized onto an epoxy-functionalized COF (oCOF-TATP) by incorporating epoxy groups into the COF framework. The covalent immobilization approach increased the enzyme loading from 26 mg/g (physical adsorption) to 40 mg/g. Secondary structure analysis revealed that the covalent binding promoted a shift from the α-helix to the β-sheet structure, with a more substantial effect than physical adsorption. Molecular dynamics simulations further confirmed that the anchoring effect enhanced structural rigidity and exposed the enzyme’s active site. This led to an 8.14% increase in accessible surface area and a 7.55% rise in hydrogen bonding interactions. These structural improvements resulted in significant catalytic enhancements, with the turnover rate (kcat) and catalytic efficiency (kcat/KM) of GOx ⊂ oCOF-TATP reaching 197 and 388% of the free enzyme, respectively. In glucose oxidation assays, the immobilized enzyme exhibited 115% of the product compared to free GOx, maintaining over 95% activity after six reuse cycles and demonstrating superior stability under various conditions. The “anchoring effect” represents a novel mechanism for optimizing enzyme performance and operational stability.
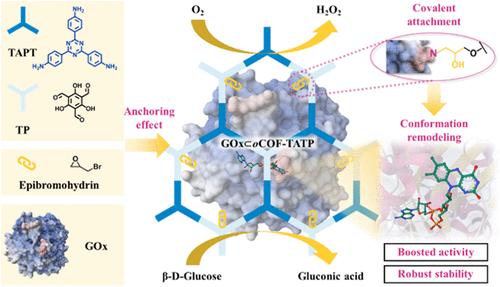
锚定效应诱导环氧功能化共价有机框架的构象重塑以提高酶效率
共价有机框架(COFs)具有结构可调和化学功能多样的特点,为固定化酶提供了广阔的应用前景。本研究探讨了共价键诱导的“锚定效应”,它能显著提高酶的效率。葡萄糖氧化酶(GOx)通过在COF框架中加入环氧基共价固定在环氧官能化COF (ocof - ttp)上。共价固定化方法将酶载量从26 mg/g(物理吸附)提高到40 mg/g。二级结构分析表明,共价结合促进了α-螺旋结构向β-片状结构的转变,其效果比物理吸附更显著。分子动力学模拟进一步证实了锚定效应增强了结构刚性,暴露了酶的活性位点。这导致可达表面积增加8.14%,氢键相互作用增加7.55%。这些结构上的改进导致了显著的催化增强,GOx∧oCOF-TATP的周转率(kcat)和催化效率(kcat/KM)分别达到游离酶的197和388%。在葡萄糖氧化实验中,与游离GOx相比,固定化酶表现出115%的产物,在6次重复使用循环后保持95%以上的活性,并在各种条件下表现出优异的稳定性。“锚定效应”代表了一种优化酶性能和操作稳定性的新机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


