Rare Ketose Synthesis from Glycolaldehyde by C2 Elongation of Aldoses Catalyzed by Transketolase Variants
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
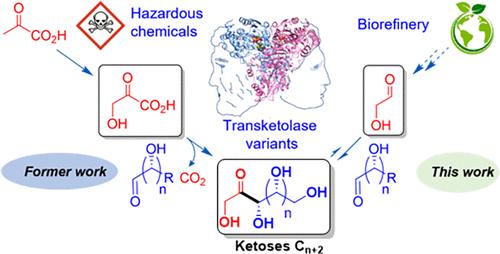
The enzyme thiamine diphosphate-dependent transketolase (TK) efficiently catalyzes the C2 elongation of aldoses using β-hydroxypyruvate (HPA) as a nucleophile for the synthesis of Cn+2 ketoses by stereoselective C–C bond formation. This synthetic approach is flawed by the tedious, low-yield chemical route to HPA. We report here an alternative transketolase-catalyzed reaction, with glycolaldehyde (GoA) as a nucleophile, for the efficient upgrading of several aldoses (C3–C5) as electrophiles, eliminating the release of carbon dioxide from the commonly used HPA. We found that the syn-acyloin condensation of GoA catalyzed by wild-type Escherichia coli transketolase (wt-TKeco) carried out under an inert atmosphere reduced byproduct formation and potentiated erythrulose (ERY) formation, giving ERY with a 78% isolated yield from 25 g·L–1 of GoA. The same reaction conditions were extended to the cross-acyloin condensation of GoA as a nucleophile with different aldoses as electrophiles, d-glyceraldehyde, l-glyceraldehyde, l-threose, d-ribose, and 5-deoxy-d-ribose, in stoichiometric amounts. Following this one-pot procedure, the corresponding Cn+2 ketoses of biological interest, d-xylulose, l-ribulose, l-sorbose, d-sedoheptulose, and 7-deoxy-d-sedoheptulose were obtained with moderate-to-high yields (38–89%) and high diastereoselectivities, showing that the TK-catalyzed reaction equilibrium was most often shifted toward the expected products. TK-catalyzed cross-acyloin reaction from GoA removes the need for HPA in the reaction, affording efficient atom economy, with product yields similar to or higher than obtained with HPA as a nucleophile.
转酮醇酶变体催化醛糖C2延伸从乙醇醛合成稀有酮糖
硫胺素二磷酸依赖转酮酶(TK)利用β-羟基丙酮酸酯(HPA)作为亲核试剂,通过立体选择的C-C键形成,有效地催化醛糖的C2延伸。这种合成方法存在缺陷,因为合成HPA的化学途径繁琐且产量低。我们在此报道了一种替代的转酮醇酶催化反应,以乙醇醛(GoA)作为亲核试剂,有效地将几种醛糖(C3-C5)升级为亲电试剂,消除了从常用的HPA中释放的二氧化碳。研究发现,野生型大肠杆菌转酮醇酶(wt-TKeco)在惰性气氛下催化果a的syna - acylloin缩合,减少了副产物的生成,增强了erythrulose (ERY)的生成,从25 g·L-1的果a中,ERY的分离收率为78%。同样的反应条件扩展到以不同醛糖为亲电试剂、d-甘油醛、l-甘油醛、l-蔗糖、d-核糖和5-脱氧-核糖的化学计量量的亲核试剂GoA的交叉酰基缩合反应。按照这个一锅法,相应的具有生物学意义的Cn+2酮糖,d-木酮糖、l-核酮糖、l-山梨糖、d-sedoheptulose和7-deoxy-d-sedoheptulose以中高收率(38-89%)和高非对映选择性得到,表明tk催化的反应平衡经常向预期产物转移。tk催化GoA的交叉丙环蛋白反应,在反应中不需要HPA,提供了高效的原子经济性,其产物收率与HPA作为亲核试剂相似或更高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


