Sterilization of Channel Catfish (Ictalurus punctatus) via Overexpression of bax Gene Regulated by a Tet-off System in the Primordial Germ Cells
Abstract
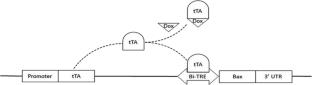
Transgenic technologies have been used for genetic improvement of catfish performance with notable success. However, these developments are useless from a commercialization standpoint without extremely efficient confinement. Transgenic sterilization has the potential to accomplish 100% reproductive confinement and avoid genetic exchange between transgenic or domestic genotypes and wild populations. The present study reports a novel sterilization method for channel catfish by overexpressing the pro-apoptosis gene bax, specifically in the primordial germ cells, to inhibit their proliferation. Three transgenic constructs were electroporated into channel catfish one-cell embryos, including Nanos-nanos, Nanos-dnd, and Dazl-vasa. Transgene integration, gonad development, and sex ratio were evaluated in P1 and F1 generations. The transgene was successfully integrated into the channel catfish genome, with variable rates depending on each construct. Mosaicism of transgene integration was widely evident in the P1 fish, as expected. All three constructs showed similar efficacy for sterilizing P1 male channel catfish, with approximately half of all males showing little to no gonadal development, resulting in a significantly lower (p < 0.05) gonadosomatic index (GSI) when compared to the control at four years of age. The same trend occurred but with lower efficacy in P1 females, with approximately one-third showing little gonadal development at four years of age. This technology is potentially useful for generating sterile male fish, where the overexpression of the bax gene can lead to reduced or no gonadal development, presumably due to the death of primordial germ cells.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


