Reflecting on interphases
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
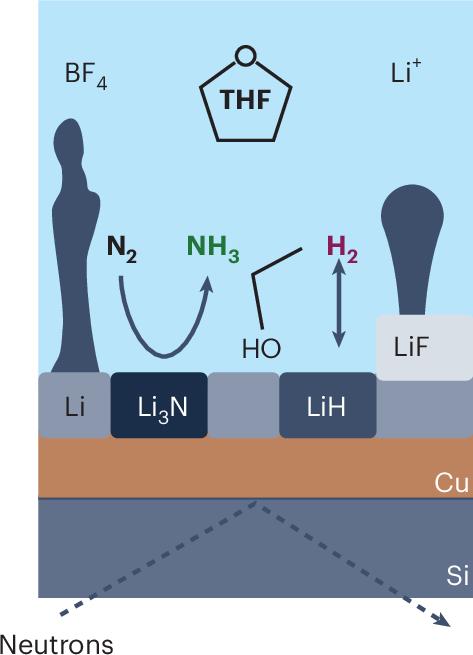
对界面的反思
中子反射计的使用是至关重要的,因为对于更常用的x射线技术来说,轻元素锂和氢实际上是不可见的。在一系列实验中揭示了关于SEI和电解质之间交换物质的一些详细见解。首先,Li盐的同一性对SEI的形成有深远的影响,在LiBF4中观察到的层比LiClO4中更明确。具体来说,该结构由厚的、扩散的外层和薄而致密的内层组成,内层在低电流密度和低循环下,在增加电流循环后合并成单层。然后,使用同位素(氘)标记溶剂(THF)和质子供体(乙醇)表明,质子供体影响内层,而溶剂控制外层。在没有质子供体的情况下,Li的枝晶生长是以牺牲Li3N和LiH为代价的。最后,中子吸收也允许在LiBF4的情况下观察到硼掺入SEI。这些见解有助于解释先前的观察结果,即锂介导的N2RR的选择性和稳定性通过使用氟化电解质得到改善。更广泛地说,这表明需要加深对SEI演变的理解,以及通过电解质添加剂进行SEI工程的能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


