The Synergistic Effect of Anion: Design of Stable Ether Electrolytes for Li Metal Batteries Beyond 4.6 V
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Improving the compatibility of ether electrolytes with high voltage condition is the key to realize high energy density lithium metal batteries (LMBs) with stable cycling, in which constructing a good cathode electrolyte interphase (CEI) is very important. Herein, a special synergistic effect of anion is found with a CEI-forming agent solvent, which significantly improves the oxidation resistance of conventional ether electrolytes. In this work, ClO4− is chosen for its strong adsorption ability, and fluoroethylene carbonate (FEC) is the CEI-forming agent. The interaction between them regulates the decomposition of anions and solvents on the cathode surface. As revealed by various tests, a thin, robust, and homogeneous CEI is generated, which ensures a stable 1,2-dimethoxyethane (DME)-based electrolyte to normally work in a Li||LiCoO2 cell for 1000 cycles at a high cut-off voltage of 4.5 V (>80% capacity retention). Differing from common approaches to enhance the oxidative stability of ether-based electrolytes, this model relies only on the synergistic effect of a simple anion (adsorbent) and film-forming agent, enabling the stable cycling of the cell using the conventional ether-based electrolytes even at 4.65 V cut-off voltage. The design strategy provides important guidelines for the implementation of ether-based LMBs with high energy density under high voltages.
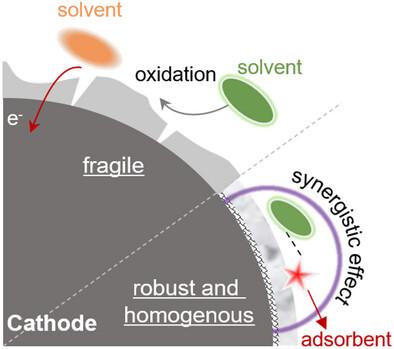
负离子的协同作用:4.6 V以上锂金属电池稳定醚电解质的设计
提高乙醚电解质与高压环境的相容性是实现高能量密度锂金属电池稳定循环的关键,而构建良好的阴极电解质界面是实现高能量密度锂金属电池稳定循环的关键。本研究发现阴离子与cei形成剂溶剂具有特殊的协同作用,显著提高了传统醚电解质的抗氧化性。本研究选择了吸附能力强的ClO4 -,氟乙烯碳酸酯(FEC)作为cei的形成剂。它们之间的相互作用调节负离子和溶剂在阴极表面的分解。各种测试表明,生成了薄、坚固、均匀的CEI,确保了1,2-二甲氧基乙烷(DME)电解质在Li||LiCoO2电池中稳定工作,在4.5 V的高截止电压下正常工作1000次(>;80%容量保留)。与提高醚基电解质氧化稳定性的常用方法不同,该模型仅依赖于简单阴离子(吸附剂)和成膜剂的协同效应,即使在4.65 V截止电压下,也能使用传统的醚基电解质稳定循环。该设计策略为实现高压下高能量密度的醚基lmb提供了重要的指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


