Interaction of Grafted Polymeric N-oxides with Charged Dyes
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
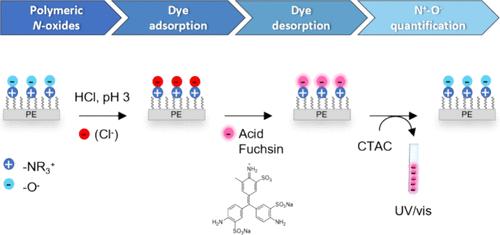
Grafted polymeric N-oxides have recently attracted interest for antifouling applications, drug delivery, wastewater purification, and electronic devices. Their function depends on the efficiency of the grafting process and the following postgrafting oxidation step. These two parameters govern the solvent-accessible charge density on the surface, an important parameter, which is notoriously hard to determine. In this study, a novel colorimetric quantitative assay for polymeric N-oxides was developed. It allows the determination of the surface charge density of grafted polymeric N-oxides. The method is based on the adsorption of acid fuchsin (AF) to grafted N-oxides through reversible electrostatic interactions between the positively charged nitrogen atoms of the N-oxide functionality and the sulfonate groups of the dye. The process depends thus on the pH-switchable properties of polymeric N-oxides. Adsorption was achieved at a pH value of 3, where N-oxides are almost fully protonated (typical pKa 4–5). AF was desorbed from the surface at pH 7 and quantified via visible adsorption spectroscopy (UV–vis) at 556 nm to determine the amount of surface-grafted functional groups. Charge densities of diverse N-oxides grafted by free radical polymerization from polyethylene (PE) were determined to be in the range 1–3 × 1015 N+-O–/cm2. Notably, N-oxides can form covalent bonds with electron-deficient triarylmethane dyes like AF. This nucleophilic reactivity of N-oxides does not compromise the proposed assay, but it may be of relevance for dye adsorption and desorption in wastewater purification.
接枝聚合物n -氧化物与带电染料的相互作用
接枝聚合物 N-氧化物最近在防污应用、药物输送、废水净化和电子设备方面引起了人们的兴趣。它们的功能取决于接枝过程和接枝后氧化步骤的效率。这两个参数控制着表面可接触溶剂的电荷密度,而这一重要参数却很难确定。本研究开发了一种新型的聚合物 N-氧化物比色定量检测方法。它可以测定接枝聚合物 N-氧化物的表面电荷密度。该方法的基础是,通过 N-氧化物官能团中带正电荷的氮原子与染料磺酸盐基团之间的可逆静电作用,酸性品红(AF)会被接枝 N-氧化物吸附。因此,这一过程取决于聚合物 N-氧化物的 pH 值可切换特性。吸附是在 pH 值为 3 时实现的,此时 N-氧化物几乎完全质子化(典型 pKa 值为 4-5)。AF 在 pH 值为 7 时从表面解吸,并通过 556 纳米可见吸附光谱(UV-vis)进行定量,以确定表面接枝官能团的数量。经测定,通过自由基聚合从聚乙烯(PE)中接枝的各种 N-氧化物的电荷密度范围为 1-3 × 1015 N+-O-/cm2。值得注意的是,N-氧化物能与缺电子的三芳基甲烷染料(如 AF)形成共价键。N-oxides 的这种亲核反应性不会影响所提出的检测方法,但它可能与废水净化中的染料吸附和解吸有关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


