Polyoxometalate-Supported Pd(II)-Catalyzed B(9)–H Nitration of o/m-Carboranes
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
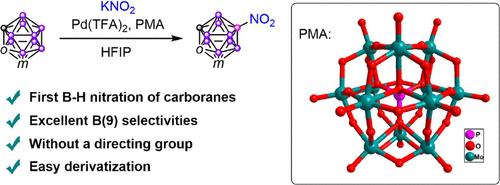
Nitro compounds are essential chemicals that serve as critical building blocks and intermediates in the synthesis of various pharmaceuticals, agrochemicals, and advanced materials. Consequently, nitration is a fundamental transformation in organic chemistry. Electrophilic aromatic substitution is one of the most important types of nitration reactions. Carboranes, a class of boron–carbon clusters characterized by their three-dimensional aromaticity, can undergo electrophilic substitution reactions. However, traditional electrophilic nitration conditions typically result in hydroxylated carboranes rather than the desired nitro compounds. Herein, we present a practical synthetic method for B(9)-NO2-o/m-carboranes via Pd(II)-catalyzed B(9)–H activation using KNO2 as the nitrating reagent, facilitated by phosphomolybdic acid (PMA). The success of this transformation hinges on the steric hindrance, strong Bro̷nsted acidity, and weak nucleophilicity of PMA, which enhance the electrophilicity of the palladium catalyst and significantly suppress the formation of B–O coupling products. Reduction of the nitro group using LiAlH4 provided 9-NH2-m-carborane, and further derivatization of 9-NO2-m-carborane and 9-NH2-m-carborane produced a series of B(9)-functionalized m-carboranes.
多金属氧酸负载Pd(II)-催化B(9) -H硝化o/m-碳硼烷
硝基化合物是合成各种药物、农用化学品和先进材料的重要组成部分和中间体。因此,硝化作用是有机化学的一个基本转变。亲电芳香族取代反应是硝化反应中最重要的一类。碳硼烷是一类具有三维芳构性的硼碳簇,可发生亲电取代反应。然而,传统的亲电硝化条件通常导致羟基化碳硼烷而不是期望的硝基化合物。在此,我们提出了一种实用的合成B(9)-NO2-o/m-碳硼烷的方法,通过Pd(II)催化B(9) -H活化,以KNO2为硝化剂,磷钼酸(PMA)促进。这一转变的成功取决于PMA的位阻、强碱性和弱亲核性,它们增强了钯催化剂的亲电性,并显著抑制了B-O偶联产物的形成。用LiAlH4还原硝基得到9- nh2 -m-碳硼烷,9- no2 -m-碳硼烷和9- nh2 -m-碳硼烷进一步衍生得到一系列B(9)功能化的间碳硼烷。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


