Enzymes in secondary pharmacology screening panels: is there room for improvement?
IF 101.8
1区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
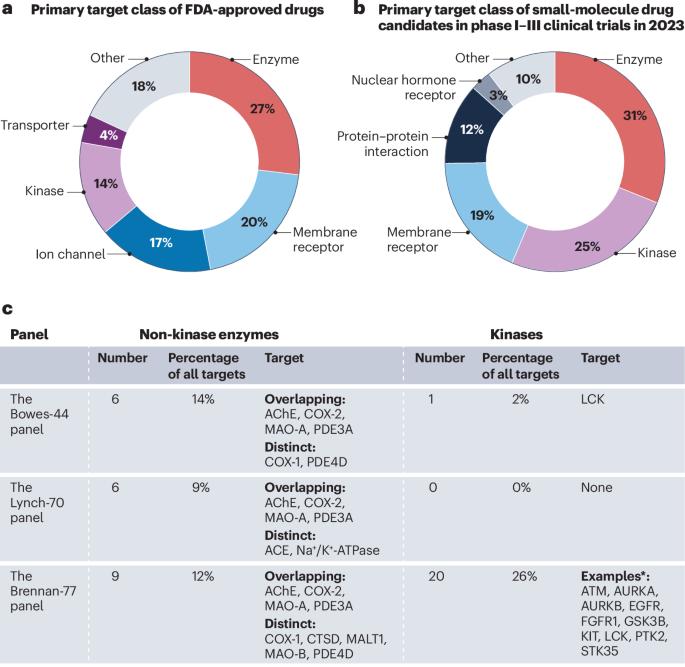

二级药理学筛选组中的酶:还有改进的空间吗?
布伦南和他的同事最近发表的一篇文章(Nat. Rev. Drug discovery . 23,525 - 545;2024)1展示了领先制药公司在应用二级药理学筛选识别候选药物脱靶活性方面的最大合作成果,表明标准化选择性分析的广泛采用对新小分子药物的安全性产生了积极影响。作者建议扩大脱靶小组,部分基于Bowes等人2和Lynch等人3提出的两个早期小组。我们赞赏布伦南及其同事完成这项重要的合作工作,并期望他们的研究将对药物开发产生重大影响。然而,我们想利用这个机会来讨论一个被忽视的问题,即迄今为止推荐的筛选小组中非激酶的表现。这三个小组包括一组部分重叠的靶点,这些靶点与明确的安全性问题有关,由44个(Bowes-44) 2,70个(Lynch-70)3和77个(Brennan-77)1靶点组成,涵盖了主要的药理学类别。在酶方面,这些图谱仅包括6 - 9种非激酶酶,占最新Brennan-77图谱中所有靶标的12%(图1c)。由于酶是“未来”和目前批准的药物的主要类别,酶在选择性面板中的份额仍然如此有限,这令人惊讶。安进最近的一项研究进一步强调了这种代表性不足,该研究显示酶参与了约三分之一的药物相关不良事件(23%的非激酶酶和10%的激酶)4。类似地,美国食品药品监督管理局对试验性新药的二级药理学研究得出结论,尽管酶的命中率相当或更高,但与其他靶标相比,酶的检测频率更低。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews. Drug Discovery
医学-生物工程与应用微生物
CiteScore
137.40
自引率
0.30%
发文量
227
期刊介绍:
Nature Reviews Drug Discovery is a monthly journal aimed at everyone working in the drug discovery and development arena.
Each issue includes:
Highest-quality reviews and perspectives covering a broad scope.
News stories investigating the hottest topics in drug discovery.
Timely summaries of key primary research papers.
Concise updates on the latest advances in areas such as new drug approvals, patent law, and emerging industry trends and strategies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


