Ligand-enabled override of the memory effect in Rh-catalyzed asymmetric Suzuki reactions
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
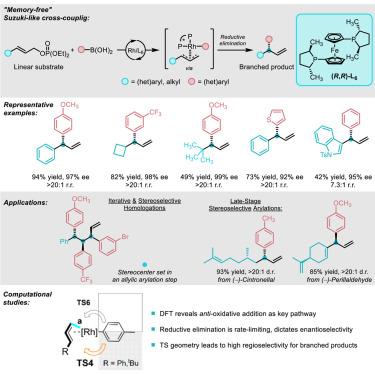
Chiral non-racemic allylic species are key building blocks for many carbon-containing molecules, including pharmaceuticals and polymers. Metal-catalyzed asymmetric additions of nucleophiles to allylic species undergo different pathways depending on the metal and nucleophile combination, hindering the development of useful addition reactions with aromatic nucleophiles. We report an asymmetric cross-coupling method between aryl boronic acids and linear allylic phosphates to give branched allylic products. This Suzuki-type reaction overcomes the “memory effect” in Rh catalysis, enabling an overall SN2′ transformation by rate-determining reductive elimination and forming a new stereogenic center adjacent to a terminal vinyl moiety. The method tolerates preexisting stereogenic centers, allowing for synthetic strategies where drugs and natural products are elaborated via diastereoselective allylic arylations. The method is used, as the catalyst-controlled stereochemistry-setting step, in an iterative strategy to give arrays of aryl-substituted contiguous stereogenic centers. This approach will complement existing catalyst-controlled stereoselective methods for forming C–C bonds.

铑催化的不对称铃木反应中配体激活的记忆效应
手性非外消旋烯丙基化合物是许多含碳分子的关键组成部分,包括药物和聚合物。金属催化的亲核试剂在烯丙基上的不对称加成反应根据金属和亲核试剂的组合而有不同的途径,阻碍了与芳香族亲核试剂的有效加成反应的发展。我们报道了芳基硼酸与线性烯丙基磷酸盐之间的不对称交叉偶联方法,以得到支链烯丙基产物。这种铃木型反应克服了Rh催化中的“记忆效应”,通过速率决定的还原消除实现SN2’的整体转化,并在末端乙烯基部分附近形成一个新的立体中心。该方法耐受预先存在的立体中心,允许通过非对映选择性烯丙基芳基化来阐述药物和天然产物的合成策略。该方法被用作催化剂控制的立体化学设置步骤,在迭代策略中得到芳基取代的连续立体中心阵列。这种方法将补充现有的催化控制的立体选择方法来形成C-C键。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


