Nickel molybdate nanorod array as a dual-function electrocatalyst for urea oxidation and hydrogen evolution in urea-assisted water splitting
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In the urea-assisted water splitting, the superhydrophilic and superaerophobic NiMoO₄ nanoarray catalyst can significantly enhance both the urea oxidation reaction (UOR) and the hydrogen evolution reaction (HER). In contrast, the aggregated Ni(OH)₂ catalyst promotes UOR but reduces HER kinetics in the presence of urea. Coral-like NiMoO₄ nanorods with mixed α and β phases can be supported on nickel foam (NF) under hydrothermal conditions with phosphomolybdic acid. Conversely, α-Ni(OH)₂ forms aggregated microspheres on NF without it. A synergistic effect between Ni and Mo modulates the electronic structure of NiMoO4 that enhances the catalytic activity, superhydrophilicity, and electrical conductivity. The NF@NiMoO4 exhibits superior catalytic performance, as demonstrated by its low Tafel slope of 14.7 mV dec⁻¹ for UOR and 77.3 mV dec⁻¹ in magnitude for HER. The hydrated NF@NiMoO₄ nanoarray enhances wettability and increases the active surface area for catalytic reactions in UOR and HER compared to NF@Ni(OH)₂. Its configuration provides a superaerophobic surface and enables rapid transport of electrons, electrolytes, and gas bubbles. Consequently, NF@NiMoO₄ offers abundant active sites, strong substrate interactions, and efficient gas release, making it a promising dual-function electrocatalyst for urea-assisted water splitting.
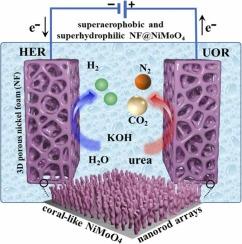
钼酸镍纳米棒阵列作为尿素氧化和尿素辅助水分离中氢进化的双重功能电催化剂
超亲水性和超疏水性NiMoO₄纳米阵列催化剂可显著促进尿素氧化反应(UOR)和析氢反应(HER)。相反,聚集的Ni(OH) 2催化剂促进了UOR,但在尿素存在下降低了HER动力学。在水热条件下,用磷酸钼酸在泡沫镍(NF)上负载具有α和β相混合的珊瑚状NiMoO₄纳米棒。相反,α-Ni(OH) 2在NF上形成聚集微球。Ni和Mo之间的协同效应调节了NiMoO4的电子结构,从而提高了催化活性、超亲水性和导电性。NF@NiMoO4具有优异的催化性能,其低的Tafel斜率(UOR为14.7 mV dec⁻¹)和HER为77.3 mV dec⁻¹。与NF@Ni(OH) 2相比,水合NF@NiMoO (OH) 2纳米阵列提高了UOR和HER催化反应的润湿性和活性表面积。它的结构提供了一个超疏氧表面,使电子、电解质和气泡能够快速传输。因此,NF@NiMoO₄具有丰富的活性位点,强底物相互作用和高效的气体释放,使其成为尿素辅助水分解的有前途的双功能电催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


